Toward the Development of Epigenome Editing-Based Therapeutics: Potentials and Challenges
- PMID: 36902207
- PMCID: PMC10003136
- DOI: 10.3390/ijms24054778
Toward the Development of Epigenome Editing-Based Therapeutics: Potentials and Challenges
Abstract
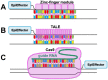
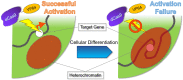
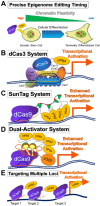
The advancement in epigenetics research over the past several decades has led to the potential application of epigenome-editing technologies for the treatment of various diseases. In particular, epigenome editing is potentially useful in the treatment of genetic and other related diseases, including rare imprinted diseases, as it can regulate the expression of the epigenome of the target region, and thereby the causative gene, with minimal or no modification of the genomic DNA. Various efforts are underway to successfully apply epigenome editing in vivo, such as improving target specificity, enzymatic activity, and drug delivery for the development of reliable therapeutics. In this review, we introduce the latest findings, summarize the current limitations and future challenges in the practical application of epigenome editing for disease therapy, and introduce important factors to consider, such as chromatin plasticity, for a more effective epigenome editing-based therapy.
Keywords: chromatin; chromatin plasticity; drug delivery; epigenetics; epigenome editing; in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

