Catechin versus MoS2 Nanoflakes Functionalized with Catechin: Improving the Sperm Fertilizing Ability-An In Vitro Study in a Swine Model
- PMID: 36902221
- PMCID: PMC10003105
- DOI: 10.3390/ijms24054788
Catechin versus MoS2 Nanoflakes Functionalized with Catechin: Improving the Sperm Fertilizing Ability-An In Vitro Study in a Swine Model
Abstract
Nowadays, the adoption of In Vitro Fertilization (IVF) techniques is undergoing an impressive increase. In light of this, one of the most promising strategies is the novel use of non-physiological materials and naturally derived compounds for advanced sperm preparation methods. Here, sperm cells were exposed during capacitation to MoS2/Catechin nanoflakes and catechin (CT), a flavonoid with antioxidant properties, at concentrations of 10, 1, 0.1 ppm. The results showed no significant differences in terms of sperm membrane modifications or biochemical pathways among the groups, allowing the hypothesis that MoS2/CT nanoflakes do not induce any negative effect on the parameters evaluated related to sperm capacitation. Moreover, the addition of CT alone at a specific concentration (0.1 ppm) increased the spermatozoa fertilizing ability in an IVF assay by increasing the number of fertilized oocytes with respect to the control group. Our findings open interesting new perspectives regarding the use of catechins and new materials obtained using natural or bio compounds, which could be used to implement the current strategies for sperm capacitation.
Keywords: catechins; in vitro fertilization; molybdenum disulfide; sperm capacitation; spermatozoa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
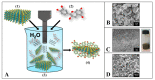
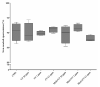
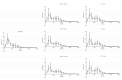
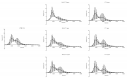

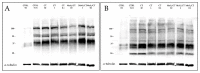

References
-
- Bernabò N., Fontana A., Sanchez M.R., Valbonetti L., Capacchietti G., Zappacosta R., Greco L., Marchisio M., Lanuti P., Ercolino E., et al. Graphene oxide affects in vitro fertilization outcome by interacting with sperm membrane in an animal model. Carbon. 2018;129:428–437. doi: 10.1016/j.carbon.2017.12.042. - DOI
-
- Bernabò N., Valbonetti L., Raspa M., Fontana A., Palestini P., Botto L.M., Paoletti R., Fray M., Allen S., Machado-Simoes J.S., et al. Graphene Oxide Improves In Vitro Fertilization in Mice with no Impact on Embryo Development and Preserves the Membrane Microdomains Architecture. Front. Bioeng. Biotechnol. 2020;8:629. doi: 10.3389/fbioe.2020.00629. - DOI - PMC - PubMed
-
- Ramal-Sanchez M., Valbonetti L., Tsikis G., Dubuisson F., Blache M.-C., Labas V., Druart X., Fontana A., Mermillod P., Barboni B., et al. Graphene oxide: A glimmer of hope for Assisted Reproductive Technology. Carbon. 2019;150:518–530. doi: 10.1016/j.carbon.2019.05.055. - DOI
-
- Rojas D., Della Pelle F., Del Carlo M., Compagnone D., Escarpa A. Group VI transition metal dichalcogenides as antifouling transducers for electrochemical oxidation of catechol-containing structures. Electrochem. Commun. 2020;115:106718. doi: 10.1016/j.elecom.2020.106718. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

