Effective, Rapid, and Small-Scale Bioconjugation and Purification of "Clicked" Small-Molecule DNA Oligonucleotide for Nucleic Acid Nanoparticle Functionalization
- PMID: 36902228
- PMCID: PMC10003352
- DOI: 10.3390/ijms24054797
Effective, Rapid, and Small-Scale Bioconjugation and Purification of "Clicked" Small-Molecule DNA Oligonucleotide for Nucleic Acid Nanoparticle Functionalization
Abstract
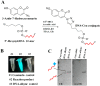
Nucleic acid-based therapeutics involves the conjugation of small molecule drugs to nucleic acid oligomers to surmount the challenge of solubility, and the inefficient delivery of these drug molecules into cells. "Click" chemistry has become popular conjugation approach due to its simplicity and high conjugation efficiency. However, the major drawback of the conjugation of oligonucleotides is the purification of the products, as traditionally used chromatography techniques are usually time-consuming and laborious, requiring copious quantities of materials. Herein, we introduce a simple and rapid purification methodology to separate the excess of unconjugated small molecules and toxic catalysts using a molecular weight cut-off (MWCO) centrifugation approach. As proof of concept, we deployed "click" chemistry to conjugate a Cy3-alkyne moiety to an azide-functionalized oligodeo-xynucleotide (ODN), as well as a coumarin azide to an alkyne-functionalized ODN. The calculated yields of the conjugated products were found to be 90.3 ± 0.4% and 86.0 ± 1.3% for the ODN-Cy3 and ODN-coumarin, respectively. Analysis of purified products by fluorescence spectroscopy and gel shift assays demonstrated a drastic amplitude of fluorescent intensity by multiple folds of the reporter molecules within DNA nanoparticles. This work is intended to demonstrate a small-scale, cost-effective, and robust approach to purifying ODN conjugates for nucleic acid nanotechnology applications.
Keywords: ODN conjugate purification; bioconjugation; click chemistry; fluorescence reporters; nucleic acid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chandler M., Johnson B., Khisamutdinov E., Dobrovolskaia M.A., Sztuba-Solinska J., Salem A.K., Breyne K., Chammas R., Walter N.G., Contreras L.M., et al. The International Society of RNA Nanotechnology and Nanomedicine (ISRNN): The Present and Future of the Burgeoning Field. ACS Nano. 2021;15:16957–16973. doi: 10.1021/acsnano.0c10240. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

