Transfer of Proteins from Cultured Human Adipose to Blood Cells and Induction of Anabolic Phenotype Are Controlled by Serum, Insulin and Sulfonylurea Drugs
- PMID: 36902257
- PMCID: PMC10003403
- DOI: 10.3390/ijms24054825
Transfer of Proteins from Cultured Human Adipose to Blood Cells and Induction of Anabolic Phenotype Are Controlled by Serum, Insulin and Sulfonylurea Drugs
Abstract
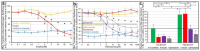
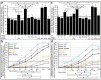
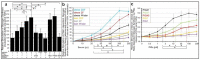
Glycosylphosphatidylinositol-anchored proteins (GPI-APs) are anchored at the outer leaflet of eukaryotic plasma membranes (PMs) only by carboxy-terminal covalently coupled GPI. GPI-APs are known to be released from the surface of donor cells in response to insulin and antidiabetic sulfonylureas (SUs) by lipolytic cleavage of the GPI or upon metabolic derangement as full-length GPI-APs with the complete GPI attached. Full-length GPI-APs become removed from extracellular compartments by binding to serum proteins, such as GPI-specific phospholipase D (GPLD1), or insertion into the PMs of acceptor cells. Here, the interplay between the lipolytic release and intercellular transfer of GPI-APs and its potential functional impact was studied using transwell co-culture with human adipocytes as insulin-/SU-responsive donor cells and GPI-deficient erythroleukemia as acceptor cells (ELCs). Measurement of the transfer as the expression of full-length GPI-APs at the ELC PMs by their microfluidic chip-based sensing with GPI-binding α-toxin and GPI-APs antibodies and of the ELC anabolic state as glycogen synthesis upon incubation with insulin, SUs and serum yielded the following results: (i) Loss of GPI-APs from the PM upon termination of their transfer and decline of glycogen synthesis in ELCs, as well as prolongation of the PM expression of transferred GPI-APs upon inhibition of their endocytosis and upregulated glycogen synthesis follow similar time courses. (ii) Insulin and SUs inhibit both GPI-AP transfer and glycogen synthesis upregulation in a concentration-dependent fashion, with the efficacies of the SUs increasing with their blood glucose-lowering activity. (iii) Serum from rats eliminates insulin- and SU-inhibition of both GPI-APs' transfer and glycogen synthesis in a volume-dependent fashion, with the potency increasing with their metabolic derangement. (iv) In rat serum, full-length GPI-APs bind to proteins, among them (inhibited) GPLD1, with the efficacy increasing with the metabolic derangement. (v) GPI-APs are displaced from serum proteins by synthetic phosphoinositolglycans and then transferred to ELCs with accompanying stimulation of glycogen synthesis, each with efficacies increasing with their structural similarity to the GPI glycan core. Thus, both insulin and SUs either block or foster transfer when serum proteins are depleted of or loaded with full-length GPI-APs, respectively, i.e., in the normal or metabolically deranged state. The transfer of the anabolic state from somatic to blood cells over long distance and its "indirect" complex control by insulin, SUs and serum proteins support the (patho)physiological relevance of the intercellular transfer of GPI-APs.
Keywords: (G)PI-specific phospholipase D (GPLD1); diabetes; glimepiride; glycosylphosphatidylinositol (GPI)-anchored proteins (GPI-APs); insulin action; protein transfer; sulfonylurea drugs (SUs).
Conflict of interest statement
The authors declare no conflict of interest (financial or otherwise).
Figures







Similar articles
-
(Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins I: Localization at Plasma Membranes and Extracellular Compartments.Biomolecules. 2023 May 18;13(5):855. doi: 10.3390/biom13050855. Biomolecules. 2023. PMID: 37238725 Free PMC article. Review.
-
(Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins II: Intercellular Transfer of Matter (Inheritance?) That Matters.Biomolecules. 2023 Jun 15;13(6):994. doi: 10.3390/biom13060994. Biomolecules. 2023. PMID: 37371574 Free PMC article. Review.
-
Biological Role of the Intercellular Transfer of Glycosylphosphatidylinositol-Anchored Proteins: Stimulation of Lipid and Glycogen Synthesis.Int J Mol Sci. 2022 Jul 4;23(13):7418. doi: 10.3390/ijms23137418. Int J Mol Sci. 2022. PMID: 35806423 Free PMC article.
-
Chip-Based Sensing of the Intercellular Transfer of Cell Surface Proteins: Regulation by the Metabolic State.Biomedicines. 2021 Oct 13;9(10):1452. doi: 10.3390/biomedicines9101452. Biomedicines. 2021. PMID: 34680568 Free PMC article.
-
Age-dependent membrane release and degradation of full-length glycosylphosphatidylinositol-anchored proteins in rats.Mech Ageing Dev. 2020 Sep;190:111307. doi: 10.1016/j.mad.2020.111307. Epub 2020 Jul 3. Mech Ageing Dev. 2020. PMID: 32628941
Cited by
-
A "poly-matter network" conception of biological inheritance.Genetica. 2024 Dec;152(4-6):211-230. doi: 10.1007/s10709-024-00216-1. Epub 2024 Oct 19. Genetica. 2024. PMID: 39425866 Free PMC article. Review.
-
(Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins I: Localization at Plasma Membranes and Extracellular Compartments.Biomolecules. 2023 May 18;13(5):855. doi: 10.3390/biom13050855. Biomolecules. 2023. PMID: 37238725 Free PMC article. Review.
-
(Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins II: Intercellular Transfer of Matter (Inheritance?) That Matters.Biomolecules. 2023 Jun 15;13(6):994. doi: 10.3390/biom13060994. Biomolecules. 2023. PMID: 37371574 Free PMC article. Review.
-
The Transformation Experiment of Frederick Griffith II: Inclusion of Cellular Heredity for the Creation of Novel Microorganisms.Bioengineering (Basel). 2025 May 15;12(5):532. doi: 10.3390/bioengineering12050532. Bioengineering (Basel). 2025. PMID: 40428151 Free PMC article. Review.
-
The Transformation Experiment of Frederick Griffith I: Its Narrowing and Potential for the Creation of Novel Microorganisms.Bioengineering (Basel). 2025 Mar 20;12(3):324. doi: 10.3390/bioengineering12030324. Bioengineering (Basel). 2025. PMID: 40150788 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

