Anterior and Posterior Tongue Regions and Taste Papillae: Distinct Roles and Regulatory Mechanisms with an Emphasis on Hedgehog Signaling and Antagonism
- PMID: 36902260
- PMCID: PMC10002505
- DOI: 10.3390/ijms24054833
Anterior and Posterior Tongue Regions and Taste Papillae: Distinct Roles and Regulatory Mechanisms with an Emphasis on Hedgehog Signaling and Antagonism
Abstract
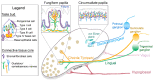
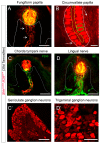
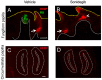
Sensory receptors across the entire tongue are engaged during eating. However, the tongue has distinctive regions with taste (fungiform and circumvallate) and non-taste (filiform) organs that are composed of specialized epithelia, connective tissues, and innervation. The tissue regions and papillae are adapted in form and function for taste and somatosensation associated with eating. It follows that homeostasis and regeneration of distinctive papillae and taste buds with particular functional roles require tailored molecular pathways. Nonetheless, in the chemosensory field, generalizations are often made between mechanisms that regulate anterior tongue fungiform and posterior circumvallate taste papillae, without a clear distinction that highlights the singular taste cell types and receptors in the papillae. We compare and contrast signaling regulation in the tongue and emphasize the Hedgehog pathway and antagonists as prime examples of signaling differences in anterior and posterior taste and non-taste papillae. Only with more attention to the roles and regulatory signals for different taste cells in distinct tongue regions can optimal treatments for taste dysfunctions be designed. In summary, if tissues are studied from one tongue region only, with associated specialized gustatory and non-gustatory organs, an incomplete and potentially misleading picture will emerge of how lingual sensory systems are involved in eating and altered in disease.
Keywords: Hedgehog antagonism; Hedgehog signaling; chorda tympani nerve; circumvallate papilla; fungiform papilla; glossopharyngeal nerve; sonidegib; taste; taste bud; taste bud progenitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation.Int J Mol Sci. 2019 Mar 16;20(6):1341. doi: 10.3390/ijms20061341. Int J Mol Sci. 2019. PMID: 30884865 Free PMC article. Review.
-
Temporal and spatial patterns of tenascin and laminin immunoreactivity suggest roles for extracellular matrix in development of gustatory papillae and taste buds.J Comp Neurol. 1996 Jan 15;364(3):535-555. doi: 10.1002/(SICI)1096-9861(19960115)364:3<535::AID-CNE11>3.0.CO;2-O. J Comp Neurol. 1996. PMID: 8820882
-
Maintenance of Taste Organs Is Strictly Dependent on Epithelial Hedgehog/GLI Signaling.PLoS Genet. 2016 Nov 28;12(11):e1006442. doi: 10.1371/journal.pgen.1006442. eCollection 2016 Nov. PLoS Genet. 2016. PMID: 27893742 Free PMC article.
-
Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium.Dev Biol. 2003 Feb 1;254(1):1-18. doi: 10.1016/s0012-1606(02)00014-3. Dev Biol. 2003. PMID: 12606278
-
Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling.Annu Rev Physiol. 2017 Feb 10;79:335-356. doi: 10.1146/annurev-physiol-022516-034202. Annu Rev Physiol. 2017. PMID: 28192057 Free PMC article. Review.
Cited by
-
Determination of objective taste perception among Iranian medical sciences students during COVID‑19 pandemic in Yazd, Eastern Iran: a case-control pilot study.BMC Infect Dis. 2024 Sep 18;24(1):997. doi: 10.1186/s12879-024-09897-7. BMC Infect Dis. 2024. PMID: 39294570 Free PMC article.
-
Distinct expression patterns of Hedgehog signaling components in mouse gustatory system during postnatal tongue development and adult homeostasis.PLoS One. 2024 Jun 7;19(6):e0294835. doi: 10.1371/journal.pone.0294835. eCollection 2024. PLoS One. 2024. PMID: 38848388 Free PMC article.
-
Regenerative potentials of bone marrow mesenchymal stem cells derived exosomes or its combination with zinc in recovery of degenerated circumvallate papilla following surgical bilateral transection of glossopharyngeal nerve in rats.BMC Oral Health. 2024 Oct 30;24(1):1320. doi: 10.1186/s12903-024-05050-7. BMC Oral Health. 2024. PMID: 39478548 Free PMC article.
-
Comparative Quantification of Fungiform Papillae Density and Taste Perception in Anemic and Healthy Controls: A Case-Control Study.Cureus. 2024 Aug 17;16(8):e67082. doi: 10.7759/cureus.67082. eCollection 2024 Aug. Cureus. 2024. PMID: 39286718 Free PMC article.
-
Variations in basic taste perception and body mass index.J Diabetes Metab Disord. 2025 May 18;24(1):122. doi: 10.1007/s40200-025-01628-2. eCollection 2025 Jun. J Diabetes Metab Disord. 2025. PMID: 40395402
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

