Oxidative Status Determines the Cytotoxicity of Ascorbic Acid in Human Oral Normal and Cancer Cells
- PMID: 36902281
- PMCID: PMC10002971
- DOI: 10.3390/ijms24054851
Oxidative Status Determines the Cytotoxicity of Ascorbic Acid in Human Oral Normal and Cancer Cells
Abstract
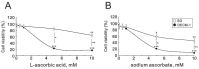
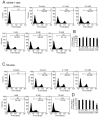
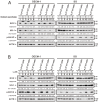
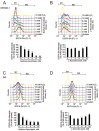
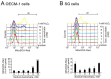
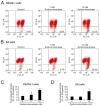
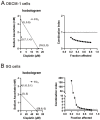
Oral squamous cell carcinoma (OSCC) can arise anywhere in the oral cavity. OSCC's molecular pathogenesis is complex, resulting from a wide range of events that involve the interplay between genetic mutations and altered levels of transcripts, proteins, and metabolites. Platinum-based drugs are the first-line treatment for OSCC; however, severe side-effects and resistance are challenging issues. Thus, there is an urgent clinical need to develop novel and/or combinatory therapeutics. In this study, we investigated the cytotoxic effects of pharmacological concentrations of ascorbate on two human oral cell lines, the oral epidermoid carcinoma meng-1 (OECM-1) cell and the Smulow-Glickman (SG) human normal gingival epithelial cell. Our study examined the potential functional impact of pharmacological concentrations of ascorbates on the cell-cycle profiles, mitochondrial-membrane potential, oxidative response, the synergistic effect of cisplatin, and the differential responsiveness between OECM-1 and SG cells. Two forms of ascorbate, free and sodium forms, were applied to examine the cytotoxic effect and it was found that both forms had a similar higher sensitivity to OECM-1 cells than to SG cells. In addition, our study data suggest that the determinant factor of cell density is important for ascorbate-induced cytotoxicity in OECM-1 and SG cells. Our findings further revealed that the cytotoxic effect might be mediated through the induction of mitochondrial reactive oxygen species (ROS) generation and the reduction in cytosolic ROS generation. The combination index supported the agonistic effect between sodium ascorbate and cisplatin in OECM-1 cells, but not in SG cells. In summary, our current findings provide supporting evidence for ascorbate to serve as a sensitizer for platinum-based treatment of OSCC. Hence, our work provides not only repurposing of the drug, ascorbate, but also an opportunity to decrease the side-effects of, and risk of resistance to, platinum-based treatment for OSCC.
Keywords: ascorbate; cisplatin; cytotoxicity; oral squamous cell carcinoma; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chuang S.L., Su W.W., Chen S.L., Yen A.M., Wang C.P., Fann J.C., Chiu S.Y., Lee Y.C., Chiu H.M., Chang D.C., et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer. 2017;123:1597–1609. doi: 10.1002/cncr.30517. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

