The Role of NLRP3, a Star of Excellence in Myeloproliferative Neoplasms
- PMID: 36902299
- PMCID: PMC10003372
- DOI: 10.3390/ijms24054860
The Role of NLRP3, a Star of Excellence in Myeloproliferative Neoplasms
Abstract
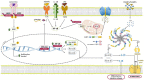
Nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) is the most widely investigated inflammasome member whose overactivation can be a driver of several carcinomas. It is activated in response to different signals and plays an important role in metabolic disorders and inflammatory and autoimmune diseases. NLRP3 belongs to the pattern recognition receptors (PRRs) family, expressed in numerous immune cells, and it plays its primary function in myeloid cells. NLRP3 has a crucial role in myeloproliferative neoplasms (MPNs), considered to be the diseases best studied in the inflammasome context. The investigation of the NLRP3 inflammasome complex is a new horizon to explore, and inhibiting IL-1β or NLRP3 could be a helpful cancer-related therapeutic strategy to improve the existing protocols.
Keywords: inflammasome; myeloproliferative neoplasms; nucleotide-binding domain (NOD)-like receptor protein 3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

