Understanding the Continuum between High-Risk Myelodysplastic Syndrome and Acute Myeloid Leukemia
- PMID: 36902450
- PMCID: PMC10002503
- DOI: 10.3390/ijms24055018
Understanding the Continuum between High-Risk Myelodysplastic Syndrome and Acute Myeloid Leukemia
Abstract
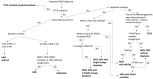
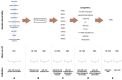
Myelodysplastic syndrome (MDS) is a clonal hematopoietic neoplasm characterized by bone marrow dysplasia, failure of hematopoiesis and variable risk of progression to acute myeloid leukemia (AML). Recent large-scale studies have demonstrated that distinct molecular abnormalities detected at earlier stages of MDS alter disease biology and predict progression to AML. Consistently, various studies analyzing these diseases at the single-cell level have identified specific patterns of progression strongly associated with genomic alterations. These pre-clinical results have solidified the conclusion that high-risk MDS and AML arising from MDS or AML with MDS-related changes (AML-MRC) represent a continuum of the same disease. AML-MRC is distinguished from de novo AML by the presence of certain chromosomal abnormalities, such as deletion of 5q, 7/7q, 20q and complex karyotype and somatic mutations, which are also present in MDS and carry crucial prognostic implications. Recent changes in the classification and prognostication of MDS and AML by the International Consensus Classification (ICC) and the World Health Organization (WHO) reflect these advances. Finally, a better understanding of the biology of high-risk MDS and the mechanisms of disease progression have led to the introduction of novel therapeutic approaches, such as the addition of venetoclax to hypomethylating agents and, more recently, triplet therapies and agents targeting specific mutations, including FLT3 and IDH1/2. In this review, we analyze the pre-clinical data supporting that high-risk MDS and AML-MRC share the same genetic abnormalities and represent a continuum, describe the recent changes in the classification of these neoplasms and summarize the advances in the management of patients with these neoplasms.
Keywords: AML with MDS-related changes; MDS; classification; molecular alterations; therapeutic approaches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

