Investigation of Cross-Linked Chitosan-Based Membranes as Potential Adsorbents for the Removal of Cu2+ Ions from Aqueous Solutions
- PMID: 36903041
- PMCID: PMC10004399
- DOI: 10.3390/ma16051926
Investigation of Cross-Linked Chitosan-Based Membranes as Potential Adsorbents for the Removal of Cu2+ Ions from Aqueous Solutions
Abstract
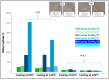
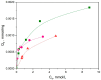
Rapid industrialization has led to huge amounts of organic pollutants and toxic heavy metals into aquatic environment. Among the different strategies explored, adsorption remains until the most convenient process for water remediation. In the present work, novel cross-linked chitosan-based membranes were elaborated as potential adsorbents of Cu2+ ions, using as cross-linking agent a random water-soluble copolymer P(DMAM-co-GMA) of glycidyl methacrylate (GMA) and N,N-dimethylacrylamide (DMAM). Cross-linked polymeric membranes were prepared through casting aqueous solutions of mixtures of P(DMAM-co-GMA) and chitosan hydrochloride, followed by thermal treatment at 120 °C. After deprotonation, the membranes were further explored as potential adsorbents of Cu2+ ions from aqueous CuSO4 solution. The successful complexation of copper ions with unprotonated chitosan was verified visually through the color change of the membranes and quantified through UV-vis spectroscopy. Cross-linked membranes based on unprotonated chitosan adsorb Cu2+ ions efficiently and decrease the concentration of Cu2+ ions in water to a few ppm. In addition, they can act as simple visual sensors for the detection of Cu2+ ions at low concentrations (~0.2 mM). The adsorption kinetics were well-described by a pseudo-second order and intraparticle diffusion model, while the adsorption isotherms followed the Langmuir model, revealing maximum adsorption capacities in the range of 66-130 mg/g. Finally, it was shown that the membranes can be effectively regenerated using aqueous H2SO4 solution and reused.
Keywords: adsorption capacity; chemical cross-linking; chitosan; copolymers; copper sulfate; glycidyl methacrylate; polymeric membranes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Ioelovich M. In: Chitosan: Derivatives, Composites and Applications. Ahmed S., Ikram S., editors. Willey; Hoboken, NJ, USA: 2017. - DOI
-
- Li Y.-Y., Wang L., Ma M.-G., Wang B. Review of recent development on preparation, properties and applications of cellulose-based functional materials. Int. J. Polym. Sci. 2018;2018:8973643. doi: 10.1155/2018/8973643. - DOI
LinkOut - more resources
Full Text Sources

