Probing the Use of Homemade Carbon Fiber Microsensor for Quantifying Caffeine in Soft Beverages
- PMID: 36903043
- PMCID: PMC10004175
- DOI: 10.3390/ma16051928
Probing the Use of Homemade Carbon Fiber Microsensor for Quantifying Caffeine in Soft Beverages
Abstract
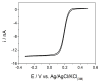
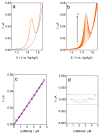
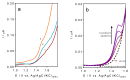
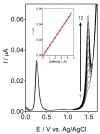
In the development of electrochemical sensors, carbon micro-structured or micro-materials have been widely used as supports/modifiers to improve the performance of bare electrodes. In the case of carbon fibers (CFs), these carbonaceous materials have received extensive attention and their use has been proposed in a variety of fields. However, to the best of our knowledge, no attempts for electroanalytical determination of caffeine with CF microelectrode (µE) have been reported in the literature. Therefore, a homemade CF-µE was fabricated, characterized, and used to determine caffeine in soft beverage samples. From the electrochemical characterization of the CF-µE in K3Fe(CN)6 10 mmol L-1 plus KCl 100 mmol L-1, a radius of about 6 µm was estimated, registering a sigmoidal voltammetric profile that distinguishes a µE indicating that the mass-transport conditions were improved. Voltammetric analysis of the electrochemical response of caffeine at the CF-µE clearly showed that no effects were attained due to the mass transport in solution. Differential pulse voltammetric analysis using the CF-µE was able to determine the detection sensitivity, concentration range (0.3 to 4.5 µmol L-1), limit of detection (0.13 μmol L-1) and linear relationship (I (µA) = (11.6 ± 0.09) × 10-3 [caffeine, μmol L-1] - (0.37 ± 0.24) × 10-3), aiming at the quantification applicability in concentration quality-control for the beverages industry. When the homemade CF-µE was used to quantify the caffeine concentration in the soft beverage samples, the values obtained were satisfactory in comparison with the concentrations reported in the literature. Additionally, the concentrations were analytically determined by high-performance liquid chromatography (HPLC). These results show that these electrodes may be an alternative to the development of new and portable reliable analytical tools at low cost with high efficiency.
Keywords: beverages; caffeine; carbon fiber; cyclic voltammetry; microelectrode.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
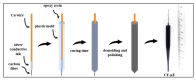

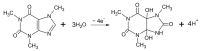
References
-
- Araujo D., Brito C., de Oliveira S.D., Silva D., Martinez-Huitle C., Aragao C. Platinum sensor for quantifying caffeine in drug formulations. Curr. Pharm. Anal. 2014;10:231–238. doi: 10.2174/1573412910666140630191329. - DOI
-
- Rostagno M.A., Manchón N., D’Arrigo M., Guillamón E., Villares A., García-Lafuente A., Ramos A., Martínez J.A. Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Anal. Chim. Acta. 2011;685:204–211. doi: 10.1016/j.aca.2010.11.031. - DOI - PubMed
-
- Rajabi Khorrami A., Rashidpur A. Development of a fiber coating based on molecular sol-gel imprinting technology for selective solid-phase micro extraction of caffeine from human serum and determination by gas chromatography/mass spectrometry. Anal. Chim. Acta. 2012;727:20–25. doi: 10.1016/j.aca.2012.03.048. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

