Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release
- PMID: 36903120
- PMCID: PMC10004356
- DOI: 10.3390/ma16052009
Particle and Phase Analysis of Combusted Iron Particles for Energy Storage and Release
Abstract
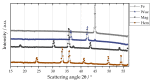
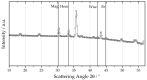
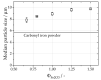
The combustion of metal fuels as energy carriers in a closed-cycle carbon-free process is a promising approach for reducing CO2 emissions in the energy sector. For a possible large-scale implementation, the influence of process conditions on particle properties and vice versa has to be well understood. In this study, the influence of different fuel-air equivalence ratios on particle morphology, size and degree of oxidation in an iron-air model burner is investigated by means of small- and wide-angle X-ray scattering, laser diffraction analysis and electron microscopy. The results show a decrease in median particle size and an increase in the degree of oxidation for leaner combustion conditions. The difference of 1.94 μm in median particle size between lean and rich conditions is twentyfold greater than the expected amount and can be connected to an increased intensity of microexplosions and nanoparticle formation for oxygen-rich atmospheres. Furthermore, the influence of the process conditions on the fuel usage efficiency is investigated, yielding efficiencies of up to 0.93. Furthermore, by choosing a suitable particle size range of 1 to 10 μm, the amount of residual iron content can be minimized. The results emphasize that particle size plays a key role in optimizing this process for the future.
Keywords: iron combustion; metal fuels; microexplosions; nanoparticles; particle characterization; small-angle X-ray scattering (SAXS); wide-angle X-ray scattering (WAXS).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Hunt J., Zakeri B., Lopes R., Barbosa P., Nascimento A., de Castro N., Brandão R., Schneider P., Wada Y. Existing and new arrangements of pumped-hydro storage plants. Renew. Sustain. Energy Rev. 2020;129:109914. doi: 10.1016/j.rser.2020.109914. - DOI
-
- Steinmann W.-D., Jockenhöfer H., Bauer D. Thermodynamic Analysis of High-Temperature Carnot Battery Concepts. Energy Technol. 2020;8:1900895. doi: 10.1002/ente.201900895. - DOI
-
- Yao Y., Lei J., Shi Y., Ai F., Lu Y.-C. Assessment methods and performance metrics for redox flow batteries. Nat. Energy. 2021;6:582–588. doi: 10.1038/s41560-020-00772-8. - DOI
-
- Bergthorson J. Recyclable metal fuels for clean and compact zero-carbon power. Prog. Energy Combust. Sci. 2018;68:169–196. doi: 10.1016/j.pecs.2018.05.001. - DOI
-
- Debiagi P., Rocha R., Scholtissek A., Janicka J., Hasse C. Iron as a sustainable chemical carrier of renewable energy: Analysis of opportunities and challenges for retrofitting coal-fired power plants. Renew. Sustain. Energy Rev. 2022;165:112579. doi: 10.1016/j.rser.2022.112579. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

