Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS)
- PMID: 36903205
- PMCID: PMC10003971
- DOI: 10.3390/ma16052090
Review of Recent Advances in Gas-Assisted Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry (FIB-TOF-SIMS)
Abstract
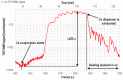
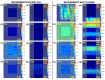
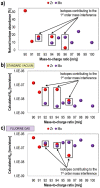
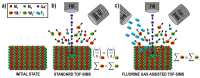
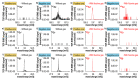
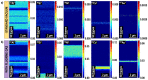
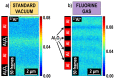
Time-of-flight secondary ion mass spectrometry (TOF-SIMS) is a powerful chemical characterization technique allowing for the distribution of all material components (including light and heavy elements and molecules) to be analyzed in 3D with nanoscale resolution. Furthermore, the sample's surface can be probed over a wide analytical area range (usually between 1 µm2 and 104 µm2) providing insights into local variations in sample composition, as well as giving a general overview of the sample's structure. Finally, as long as the sample's surface is flat and conductive, no additional sample preparation is needed prior to TOF-SIMS measurements. Despite many advantages, TOF-SIMS analysis can be challenging, especially in the case of weakly ionizing elements. Furthermore, mass interference, different component polarity of complex samples, and matrix effect are the main drawbacks of this technique. This implies a strong need for developing new methods, which could help improve TOF-SIMS signal quality and facilitate data interpretation. In this review, we primarily focus on gas-assisted TOF-SIMS, which has proven to have potential for overcoming most of the aforementioned difficulties. In particular, the recently proposed use of XeF2 during sample bombardment with a Ga+ primary ion beam exhibits outstanding properties, which can lead to significant positive secondary ion yield enhancement, separation of mass interference, and inversion of secondary ion charge polarity from negative to positive. The implementation of the presented experimental protocols can be easily achieved by upgrading commonly used focused ion beam/scanning electron microscopes (FIB/SEM) with a high vacuum (HV)-compatible TOF-SIMS detector and a commercial gas injection system (GIS), making it an attractive solution for both academic centers and the industrial sectors.
Keywords: FIB-TOF-SIMS; elemental characterization; gas injection system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Benninghoven A., Rudenauer F.G., Werner H.W. Secondary Ion Mass Spectrometry–Basic Concepts, Instrumental Aspects, Applications and Trends. Surf. Interface Anal. 1987;10:435. doi: 10.1002/sia.740100811. - DOI
-
- Van de Heide P. Secondary Ion Mass Spectrometry: An Introduction to Principles and Practices. Wiley; Hoboken, NJ, USA: 2014.
-
- Vickermann J.C., Briggs D. ToF-SIMS: Materials Analysis by Mass Spectrometry. 2nd ed. IM Publications LLP; West Sussex, UK: 2013.
-
- Priebe A., Bleuet P., Goret G., Laurencin J., Montinaro D., Barnes J.-P. State-of-the-Art Three-Dimensional Chemical Characterization of Solid Oxide Fuel Cell Using Focused Ion Beam Time-of-Flight Secondary Ion Mass Spectrometry Tomography. Microsc. Microanal. 2016;22:1261–1269. doi: 10.1017/S1431927616012502. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

