2-Amino-1,3-benzothiazole: Endo N-Alkylation with α-Iodo Methyl Ketones Followed by Cyclization
- PMID: 36903340
- PMCID: PMC10004639
- DOI: 10.3390/molecules28052093
2-Amino-1,3-benzothiazole: Endo N-Alkylation with α-Iodo Methyl Ketones Followed by Cyclization
Abstract
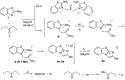
Reactions of 2-amino-1,3-benzothiazole with aliphatic, aromatic and heteroaromatic α-iodoketones in the absence of bases or catalysts have been studied. The reaction proceeds by N-alkylation of the endocyclic nitrogen atom followed by intramolecular dehydrative cyclization. The regioselectivity is explained and the mechanism of the reaction is proposed. A number of new linear and cyclic iodide and triiodide benzothiazolium salts have been obtained and their structure proved by NMR and UV spectroscopy.
Keywords: 1,3-diiodoacetone; 2-amino-1,3-benzothiazole; N-alkylation; aromatic; heteroaromatic α-iodo methyl ketones; intramolecular cyclization; iodide and triiodide salts; iodoacetone.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Sultanova R.M., Lobov A.N., Shumadalova A.V., Meshcheryakova S.A., Zileeva Z.R., Khusnutdinova N.S., Vakhitov V.A., Vakhitova Y.V. Synthesis of new 1,3-thiazole derivatives of maleopimaric acid as antitumor, antibacterial and antifungal agents. Nat. Prod. Res. 2021;35:1340–1348. doi: 10.1080/14786419.2019.1648459. - DOI - PubMed
-
- Galochkina A.V., Bollikanda R.K., Zarubaev V.V., Tentler D.G., Lavrenteva I.N., Slita A.V., Chirra N., Kantevari S. Synthesis of novel derivatives of 7,8-dihydro-6H-imidazo[2,1-b][1,3]benzothiazol-5-one and their virus-inhibiting activity against influenza A virus. Arch. Pharm. Chem. Life Sci. 2019;352:e1800225. doi: 10.1002/ardp.201800225. - DOI - PubMed
-
- Rezaei Z., Sarkari B., Khabnadideh S., Farjami M., Mehrjou M., Yazdi A., Riazimontazer E., Fararouei M. Synthesis and biological activity of some aminothiazole derivatives as anti-leishmania agents. Anti-Infect. Agents. 2020;18:178−189. doi: 10.2174/2211352517666190527112955. - DOI
LinkOut - more resources
Full Text Sources

