BF2-Azadipyrromethene Fluorophores for Intraoperative Vital Structure Identification
- PMID: 36903411
- PMCID: PMC10004488
- DOI: 10.3390/molecules28052167
BF2-Azadipyrromethene Fluorophores for Intraoperative Vital Structure Identification
Abstract
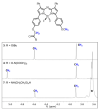
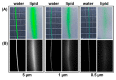
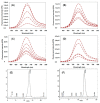
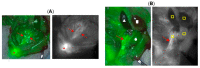
A series of mono- and bis-polyethylene glycol (PEG)-substituted BF2-azadipyrromethene fluorophores have been synthesized with emissions in the near-infrared region (700-800 nm) for the purpose of fluorescence guided intraoperative imaging; chiefly ureter imaging. The Bis-PEGylation of fluorophores resulted in higher aqueous fluorescence quantum yields, with PEG chain lengths of 2.9 to 4.6 kDa being optimal. Fluorescence ureter identification was possible in a rodent model with the preference for renal excretion notable through comparative fluorescence intensities from the ureters, kidneys and liver. Ureteral identification was also successfully performed in a larger animal porcine model under abdominal surgical conditions. Three tested doses of 0.5, 0.25 and 0.1 mg/kg all successfully identified fluorescent ureters within 20 min of administration which was sustained up to 120 min. 3-D emission heat map imaging allowed the spatial and temporal changes in intensity due to the distinctive peristaltic waves of urine being transferred from the kidneys to the bladder to be identified. As the emission of these fluorophores could be spectrally distinguished from the clinically-used perfusion dye indocyanine green, it is envisaged that their combined use could be a step towards intraoperative colour coding of different tissues.
Keywords: BF2-azadipyrromethene; NIR-fluorescence; fluorescence guided surgery; pegylation; ureter identification.
Conflict of interest statement
DFOS has a financial interest in patents filed and granted relating to NIR-fluorophores and processes for visual determination of tissue biology. RC is named on a patent filed in relation to processes for visual determination of tissue biology and receives speaker fees from Stryker Corp, consultancy fees from Distal Motion and holds research funding from Intuitive Corp and with IBM Corp and Deciphex. PMA, SZ are full-time employees of IBM Research, a division of IBM Corporation. IBM Corporation provides technical products and services world-wide to government, healthcare and life-sciences companies. PMA, SZ hold and have filed patents concerning technologies related to the subject matter of this paper. MD is member of the Advisory Board of Diagnostic Green and is the recipient of the ELIOS grant from the ARC Foundation. JM is the President of the IRCAD Institute, which is partly funded by KARL STORZ and Medtronic.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

