Bufalin-Mediated Regulation of Cell Signaling Pathways in Different Cancers: Spotlight on JAK/STAT, Wnt/β-Catenin, mTOR, TRAIL/TRAIL-R, and Non-Coding RNAs
- PMID: 36903477
- PMCID: PMC10004807
- DOI: 10.3390/molecules28052231
Bufalin-Mediated Regulation of Cell Signaling Pathways in Different Cancers: Spotlight on JAK/STAT, Wnt/β-Catenin, mTOR, TRAIL/TRAIL-R, and Non-Coding RNAs
Abstract
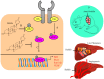
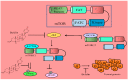
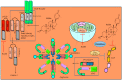
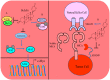
The renaissance of research into natural products has unequivocally and paradigmatically shifted our knowledge about the significant role of natural products in cancer chemoprevention. Bufalin is a pharmacologically active molecule isolated from the skin of the toad Bufo gargarizans or Bufo melanostictus. Bufalin has characteristically unique properties to regulate multiple molecular targets and can be used to harness multi-targeted therapeutic regimes against different cancers. There is burgeoning evidence related to functional roles of signaling cascades in carcinogenesis and metastasis. Bufalin has been reported to regulate pleiotropically a myriad of signal transduction cascades in various cancers. Importantly, bufalin mechanistically regulated JAK/STAT, Wnt/β-Catenin, mTOR, TRAIL/TRAIL-R, EGFR, and c-MET pathways. Furthermore, bufalin-mediated modulation of non-coding RNAs in different cancers has also started to gain tremendous momentum. Similarly, bufalin-mediated targeting of tumor microenvironments and tumor macrophages is an area of exciting research and we have only started to scratch the surface of the complicated nature of molecular oncology. Cell culture studies and animal models provide proof-of-concept for the impetus role of bufalin in the inhibition of carcinogenesis and metastasis. Bufalin-related clinical studies are insufficient and interdisciplinary researchers require detailed analysis of the existing knowledge gaps.
Keywords: apoptosis; bufalin; cancer; cell signaling; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

