Recent Advances in Asymmetric Synthesis of Pyrrolidine-Based Organocatalysts and Their Application: A 15-Year Update
- PMID: 36903480
- PMCID: PMC10005811
- DOI: 10.3390/molecules28052234
Recent Advances in Asymmetric Synthesis of Pyrrolidine-Based Organocatalysts and Their Application: A 15-Year Update
Abstract
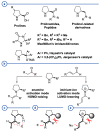
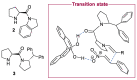
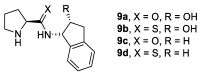
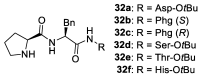
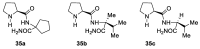
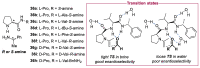
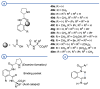
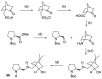
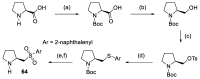
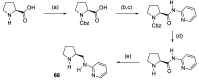
In 1971, chemists from Hoffmann-La Roche and Schering AG independently discovered a new asymmetric intramolecular aldol reaction catalyzed by the natural amino acid proline, a transformation now known as the Hajos-Parrish-Eder-Sauer-Wiechert reaction. These remarkable results remained forgotten until List and Barbas reported in 2000 that L-proline was also able to catalyze intermolecular aldol reactions with non-negligible enantioselectivities. In the same year, MacMillan reported on asymmetric Diels-Alder cycloadditions which were efficiently catalyzed by imidazolidinones deriving from natural amino acids. These two seminal reports marked the birth of modern asymmetric organocatalysis. A further important breakthrough in this field happened in 2005, when Jørgensen and Hayashi independently proposed the use of diarylprolinol silyl ethers for the asymmetric functionalization of aldehydes. During the last 20 years, asymmetric organocatalysis has emerged as a very powerful tool for the facile construction of complex molecular architectures. Along the way, a deeper knowledge of organocatalytic reaction mechanisms has been acquired, allowing for the fine-tuning of the structures of privileged catalysts or proposing completely new molecular entities that are able to efficiently catalyze these transformations. This review highlights the most recent advances in the asymmetric synthesis of organocatalysts deriving from or related to proline, starting from 2008.
Keywords: asymmetric organocatalysis; proline; substituted pyrrolidines; synthetic methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







































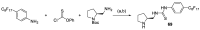




























Similar articles
-
Theory of asymmetric organocatalysis of Aldol and related reactions: rationalizations and predictions.Acc Chem Res. 2004 Aug;37(8):558-69. doi: 10.1021/ar0300524. Acc Chem Res. 2004. PMID: 15311955
-
Evaluating beta-amino acids as enantioselective organocatalysts of the Hajos-Parrish-Eder-Sauer-Wiechert reaction.Org Biomol Chem. 2007 Oct 7;5(19):3190-200. doi: 10.1039/b711171a. Epub 2007 Aug 29. Org Biomol Chem. 2007. PMID: 17878978
-
The effects of interactions between proline and carbon nanostructures on organocatalysis in the Hajos-Parrish-Eder-Sauer-Wiechert reaction.Nanoscale. 2014 Oct 7;6(19):11141-6. doi: 10.1039/c4nr04009k. Nanoscale. 2014. PMID: 25213437
-
'Five at one stroke': proline and small peptides in the stereoselective de novo synthesis and enantiotopic functionalization of carbohydrates.Chem Biodivers. 2005 Jul;2(7):825-36. doi: 10.1002/cbdv.200590061. Chem Biodivers. 2005. PMID: 17193175 Review.
-
Asymmetric cycloaddition reactions catalysed by diarylprolinol silyl ethers.Chem Soc Rev. 2017 Feb 20;46(4):1080-1102. doi: 10.1039/c6cs00713a. Chem Soc Rev. 2017. PMID: 27883141 Review.
Cited by
-
Stereoselective Synthesis of α-Disubstituted β-Homoprolines.Org Lett. 2023 Sep 29;25(38):7067-7071. doi: 10.1021/acs.orglett.3c02891. Epub 2023 Sep 20. Org Lett. 2023. PMID: 37729003 Free PMC article.
-
Skeletal Transformations Observed in the Reaction of a Tricyclic Thymine Nucleoside with Dicarbonyl Compounds.ACS Omega. 2024 Aug 12;9(34):36259-36272. doi: 10.1021/acsomega.4c02553. eCollection 2024 Aug 27. ACS Omega. 2024. PMID: 39220522 Free PMC article.
References
-
- Stocker B.L., Dangerfield E.M., Win-Mason A.L., Haslett G.W., Timmer M.S.M. Recent Developments in the Synthesis of Pyrrolidine-Containing Iminosugars. Eur. J. Org. Chem. 2010;2010:1615–1637. doi: 10.1002/ejoc.200901320. - DOI
-
- Zhou M., El-Sayed E.S.M., Ju Z., Wang W., Yuan D. The Synthesis and Applications of Chiral Pyrrolidine Functionalized Metal-Organic Frameworks and Covalent-Organic Frameworks. Inorg. Chem. Front. 2020;7:1319–1333. doi: 10.1039/C9QI01103J. - DOI
-
- Vega-Peñaloza A., Paria S., Bonchio M., Dell’Amico L., Companyó X. Profiling the Privileges of Pyrrolidine-Based Catalysts in Asymmetric Synthesis: From Polar to Light-Driven Radical Chemistry. ACS Catal. 2019;9:6058–6072. doi: 10.1021/acscatal.9b01556. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

