Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts
- PMID: 36903521
- PMCID: PMC10005273
- DOI: 10.3390/molecules28052263
Phytochemicals, Antioxidant and Antimicrobial Potentials and LC-MS Analysis of Centaurea parviflora Desf. Extracts
Abstract
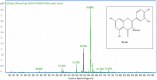
Centaurea parviflora (C. parviflora), belonging to the family Asteraceae, is an Algerian medicinal plant used in folk medicine to treat different diseases related to hyperglycemic and inflammatory disorders, as well as in food. The present study aimed to assess the total phenolic content, in vitro antioxidant and antimicrobial activity and phytochemical profile of the extracts of C. parviflora. The extraction of phenolic compounds from aerial parts was conducted using solvents of increasing polarity starting from methanol, resulting in crude extract (CE), to chloroform extract (CHE), ethyl acetate extract (EAE) and butanol extract (BUE). The total phenolic, flavonoid and flavonol contents of the extracts were determined using the Folin-Ciocalteu and AlCl3 methods, respectively. The antioxidant activity was measured with seven methods: 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, galvinoxyl free-radical-scavenging test, 2,2'-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) assay, cupric reducing antioxidant capacity (CUPRAC), reducing power, Fe+2-phenanthroline reduction assay and superoxide-scavenging test. The disc-diffusion method aimed at testing the sensitivity of bacterial strains toward our extracts. A qualitative analysis with thin-layer chromatography of the methanolic extract was performed. Moreover, HPLC-DAD-MS was used to establish the phytochemical profile of the BUE. The BUE was found to contain high amounts of total phenolics (175.27 ± 2.79 µg GAE/mg E), flavonoids (59.89 ± 0.91 µg QE/mg E) and flavonols (47.30 ± 0.51 µg RE/mg E). Using TLC, different components such as flavonoids and polyphenols were noted. The highest radical-scavenging ability was recorded for the BUE against DPPH (IC50 = 59.38 ± 0.72 µg/mL), galvinoxyl (IC50 = 36.25 ± 0.42 µg/mL), ABTS (IC50 = 49.52 ± 1.54 µg/mL) and superoxide (IC50 = 13.61 ± 0.38 µg/mL). The BUE had the best reducing power according to the CUPRAC (A0.5 = 71.80 ± 1.22 μg/mL), phenanthroline test (A0.5 = 20.29 ± 1.16 μg/mL) and FRAP (A0.5 = 119.17 ± 0.29 μg/mL). The LC-MS analysis of BUE allowed us to identify eight compounds including six phenolic acids and two flavonoids: quinic acid, five chlorogenic acid derivatives, rutin and quercetin 3-o-glucoside. This preliminary investigation revealed that the extracts of C. parviflora have a good biopharmaceutical activity. The BUE possesses an interesting potential for pharmaceutical/nutraceutical applications.
Keywords: Centaurea parviflora; LC-MS; TLC; antimicrobial; antioxidant activity; flavonoids; polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Phytochemical screening, HPLC analysis, antimicrobial and antioxidant effect of Euphorbia parviflora L. (Euphorbiaceae Juss.).Sci Rep. 2024 Mar 7;14(1):5627. doi: 10.1038/s41598-024-55905-w. Sci Rep. 2024. PMID: 38454096 Free PMC article.
-
Phytochemical Composition, Antioxidant, and Antimicrobial Attributes of Different Solvent Extracts from Myrica esculenta Buch.-Ham. ex. D. Don Leaves.Biomolecules. 2019 Aug 9;9(8):357. doi: 10.3390/biom9080357. Biomolecules. 2019. PMID: 31405047 Free PMC article.
-
Volatile and phenolic profiling of a traditional medicinal plant, Hypericum empetrifolium with in vitro biological activities.J Ethnopharmacol. 2021 May 23;272:113933. doi: 10.1016/j.jep.2021.113933. Epub 2021 Feb 15. J Ethnopharmacol. 2021. PMID: 33600919
-
Comparative Analysis of Lespedeza Species: Traditional Uses and Biological Activity of the Fabaceae Family.Molecules. 2025 Apr 30;30(9):2013. doi: 10.3390/molecules30092013. Molecules. 2025. PMID: 40363818 Free PMC article. Review.
-
Litchi flavonoids: isolation, identification and biological activity.Molecules. 2007 Apr 11;12(4):745-58. doi: 10.3390/12040745. Molecules. 2007. PMID: 17851427 Free PMC article. Review.
Cited by
-
Inhibitory Effects of Fermented Sprouted Oat Extracts on Oxidative Stress and Melanin Overproduction.Antioxidants (Basel). 2024 Apr 29;13(5):544. doi: 10.3390/antiox13050544. Antioxidants (Basel). 2024. PMID: 38790649 Free PMC article.
-
Exploring the benefits of AMF colonization for improving wheat growth, physiology and metabolism, and antimicrobial activity under biotic stress from aphid infection.BMC Plant Biol. 2025 Feb 14;25(1):198. doi: 10.1186/s12870-025-06196-4. BMC Plant Biol. 2025. PMID: 39953402 Free PMC article.
References
-
- Lawrence G.H.M. Taxonomy of Vascular Plants. Oxford and IBM Publishing Co.; New Delhi, India: 1973. Rakes Press.
-
- Matsuda H., Morikawa T., Toguchida I., Harima S., Yoshikawa M. Medicinal Flowers. VI. Absolute Stereostructures of Two New Flavanone Glycosides and a Phenylbutanoid Glycoside from the Flowers of Chrysanthemum indicum L.: Their Inhibitory Activities for Rat Lens Aldose Reductase. Chem. Pharm. Bull. 2002;50:972. doi: 10.1248/cpb.50.972. - DOI - PubMed
-
- Da Silva A.C.N., do Nascimento R.M.C., do Rodrigues D.C.N., Ferreira P.M.P., Pessoa C., Lima D.J.B., de Moraes Filho M.O., de Almeida R.M., Ferreira S.R., Fujiwara R.T., et al. In vitro activity evaluation of seven Brazilian Asteraceae against cancer cells and Leishmania amazonensis. S. Afr. J. Bot. 2019;121:267–273. doi: 10.1016/j.sajb.2018.11.008. - DOI
-
- Sarembaud A., Poitevin B. Médicament à usage homéopathique. Ed. Masson; Paris, France: 1996. p. 256.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

