Folic Acid and Leucovorin Have Potential to Prevent SARS-CoV-2-Virus Internalization by Interacting with S-Glycoprotein/Neuropilin-1 Receptor Complex
- PMID: 36903540
- PMCID: PMC10005443
- DOI: 10.3390/molecules28052294
Folic Acid and Leucovorin Have Potential to Prevent SARS-CoV-2-Virus Internalization by Interacting with S-Glycoprotein/Neuropilin-1 Receptor Complex
Abstract
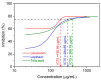
The interaction of the SARS-CoV-2 spike (S) glycoprotein receptor-binding domain with the host-cell ACE2 receptor is a well-known step in virus infection. Neuropilin-1 (NRP-1) is another host factor involved in virus internalization. The interaction between S-glycoprotein and NRP-1 has been identified as a potential COVID-19 treatment target. Herein, the effectiveness of folic acid and leucovorin in preventing contact between S-glycoprotein and NRP-1 receptors was investigated using in silico studies and then confirmed in vitro. The results of a molecular docking study showed that leucovorin and folic acid had lower binding energies than EG01377, a well-known NRP-1 inhibitor, and lopinavir. Two hydrogen bonds with Asp 320 and Asn 300 residues stabilized the leucovorin, while interactions with Gly 318, Thr 349, and Tyr 353 residues stabilized the folic acid. The molecular dynamic simulation revealed that the folic acid and leucovorin created very stable complexes with the NRP-1. The in vitro studies showed that the leucovorin was the most active inhibitor of the S1-glycoprotein/NRP-1 complex formation, with an IC75 value of 185.95 µg/mL. The results of this study suggest that folic acid and leucovorin could be considered as potential inhibitors of the S-glycoprotein/NRP-1 complex and, thus, could prevent the SARS-CoV-2 virus' entry into host cells.
Keywords: COVID-19; SARS-CoV-2; folic acid; in silico; in vitro; leucovorin; neuropilin-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

