Rational Computational Approaches in Drug Discovery: Potential Inhibitors for Allosteric Regulation of Mutant Isocitrate Dehydrogenase-1 Enzyme in Cancers
- PMID: 36903561
- PMCID: PMC10005488
- DOI: 10.3390/molecules28052315
Rational Computational Approaches in Drug Discovery: Potential Inhibitors for Allosteric Regulation of Mutant Isocitrate Dehydrogenase-1 Enzyme in Cancers
Abstract
Mutations in homodimeric isocitrate dehydrogenase (IDH) enzymes at specific arginine residues result in the abnormal activity to overproduce D-2 hydroxyglutarate (D-2HG), which is often projected as solid oncometabolite in cancers and other disorders. As a result, depicting the potential inhibitor for D-2HG formation in mutant IDH enzymes is a challenging task in cancer research. The mutation in the cytosolic IDH1 enzyme at R132H, especially, may be associated with higher frequency of all types of cancers. So, the present work specifically focuses on the design and screening of allosteric site binders to the cytosolic mutant IDH1 enzyme. The 62 reported drug molecules were screened along with biological activity to identify the small molecular inhibitors using computer-aided drug design strategies. The designed molecules proposed in this work show better binding affinity, biological activity, bioavailability, and potency toward the inhibition of D-2HG formation compare to the reported drugs in the in silico approach.
Keywords: 2-Hydroxyglutarate; 3D-QSAR; ADME; CADD; cancers; chirality; drug discovery; epigenetics; inhibitors; molecular docking; molecular dynamics simulation; oncometabolite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



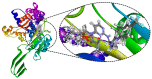
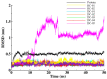
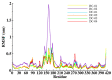

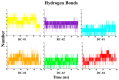


Similar articles
-
Treatment with a Small Molecule Mutant IDH1 Inhibitor Suppresses Tumorigenic Activity and Decreases Production of the Oncometabolite 2-Hydroxyglutarate in Human Chondrosarcoma Cells.PLoS One. 2015 Sep 14;10(9):e0133813. doi: 10.1371/journal.pone.0133813. eCollection 2015. PLoS One. 2015. PMID: 26368816 Free PMC article.
-
Low pH Facilitates Heterodimerization of Mutant Isocitrate Dehydrogenase IDH1-R132H and Promotes Production of 2-Hydroxyglutarate.Biochemistry. 2021 Jun 29;60(25):1983-1994. doi: 10.1021/acs.biochem.1c00059. Epub 2021 Jun 18. Biochemistry. 2021. PMID: 34143606 Free PMC article.
-
Characterisation of isocitrate dehydrogenase 1/isocitrate dehydrogenase 2 gene mutation and the d-2-hydroxyglutarate oncometabolite level in dedifferentiated chondrosarcoma.Histopathology. 2020 Apr;76(5):722-730. doi: 10.1111/his.14018. Epub 2020 Mar 10. Histopathology. 2020. PMID: 31609487
-
Targeting Isocitrate Dehydrogenase Mutations in Cancer: Emerging Evidence and Diverging Strategies.Clin Cancer Res. 2021 Jan 15;27(2):383-388. doi: 10.1158/1078-0432.CCR-20-1827. Epub 2020 Sep 3. Clin Cancer Res. 2021. PMID: 32883741 Review.
-
Isocitrate dehydrogenase variants in cancer - Cellular consequences and therapeutic opportunities.Curr Opin Chem Biol. 2020 Aug;57:122-134. doi: 10.1016/j.cbpa.2020.06.012. Epub 2020 Aug 8. Curr Opin Chem Biol. 2020. PMID: 32777735 Free PMC article. Review.
Cited by
-
Machine learning-based QSAR and structure-based virtual screening guided discovery of novel mIDH1 inhibitors from natural products.J Comput Aided Mol Des. 2025 Jul 8;39(1):44. doi: 10.1007/s10822-025-00624-1. J Comput Aided Mol Des. 2025. PMID: 40629220
-
Screening, docking, and molecular dynamics analysis of Mitragyna speciosa (Korth.) compounds for targeting HER2 in breast cancer.Curr Res Struct Biol. 2025 Jun 20;10:100171. doi: 10.1016/j.crstbi.2025.100171. eCollection 2025 Dec. Curr Res Struct Biol. 2025. PMID: 40642117 Free PMC article.
-
Identification of mIDH1 R132C/S280F Inhibitors from Natural Products by Integrated Molecular Docking, Pharmacophore Modeling and Molecular Dynamics Simulations.Pharmaceuticals (Basel). 2024 Mar 5;17(3):336. doi: 10.3390/ph17030336. Pharmaceuticals (Basel). 2024. PMID: 38543123 Free PMC article.
References
-
- Cairns R.A., Mak T.W. Oncogenic isocitrate dehydrogenase mutations: Mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3:730–741. doi: 10.1158/2159-8290.CD-13-0083. - DOI - PubMed
-
- Badur M.G., Muthusamy T., Parker S.J., Ma S., McBrayer S.K., Cordes T., Magana J.H., Guan K.L., Metallo C.M. Oncogenic R132 IDH1 Mutations Limit NADPH for De Novo Lipogenesis through (D)2-Hydroxyglutarate Production in Fibrosarcoma Sells. Cell Rep. 2018;25:1018–1026.e4. doi: 10.1016/j.celrep.2018.09.074. - DOI - PMC - PubMed
-
- Xiong Y., Xu W., Yang H., Wang P., Ma S., Zhao S., Ye D., Guan K. The mechanisms of oncometabolites in epigenetic control, DNA repair, neural development and gliomagenesis. Neurol. Oncol. 2017;19((Suppl. S3)):iii68. doi: 10.1093/neuonc/nox036.254. - DOI
-
- Thamim M., Thirumoorthy K. Computational studies of selective N-methylation in nicotinamide: Epigenetic reprogramming in cancer. Comput. Theor. Chem. 2021;1194:113058. doi: 10.1016/j.comptc.2020.113058. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

