Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population
- PMID: 36903575
- PMCID: PMC10004871
- DOI: 10.3390/molecules28052332
Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population
Abstract
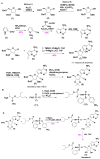
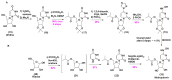
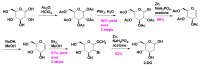
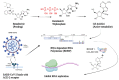
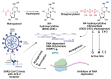
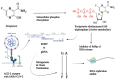
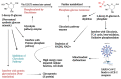
The COVID-19 pandemic has flared across every part of the globe and affected populations from different age groups differently. People aged from 40 to 80 years or older are at an increased risk of morbidity and mortality due to COVID-19. Therefore, there is an urgent requirement to develop therapeutics to decrease the risk of the disease in the aged population. Over the last few years, several prodrugs have demonstrated significant anti-SARS-CoV-2 effects in in vitro assays, animal models, and medical practice. Prodrugs are used to enhance drug delivery by improving pharmacokinetic parameters, decreasing toxicity, and attaining site specificity. This article discusses recently explored prodrugs such as remdesivir, molnupiravir, favipiravir, and 2-deoxy-D-glucose (2-DG) and their implications in the aged population, as well as investigating recent clinical trials.
Keywords: 2-Deoxy-D-glucose (2-DG); aged population; favipiravir; molnupiravir; prodrug; remdesivir.
Conflict of interest statement
The authors declare no conflicts of interest concerning the authorship and publication of this article.
Figures


Similar articles
-
Synthesis and anti-SARS-CoV-2 evaluation of lipid prodrugs of β-D-N4-hydroxycytidine (NHC) and a 3'-fluoro-substituted analogue of NHC.Bioorg Chem. 2023 Jun;135:106527. doi: 10.1016/j.bioorg.2023.106527. Epub 2023 Apr 6. Bioorg Chem. 2023. PMID: 37031504 Free PMC article.
-
Broad-spectrum prodrugs with anti-SARS-CoV-2 activities: Strategies, benefits, and challenges.J Med Virol. 2022 Apr;94(4):1373-1390. doi: 10.1002/jmv.27517. Epub 2021 Dec 18. J Med Virol. 2022. PMID: 34897729 Review.
-
Rethinking Remdesivir: Synthesis, Antiviral Activity, and Pharmacokinetics of Oral Lipid Prodrugs.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0115521. doi: 10.1128/AAC.01155-21. Epub 2021 Jul 26. Antimicrob Agents Chemother. 2021. PMID: 34310217 Free PMC article.
-
Viral target and metabolism-based rationale for combined use of recently authorized small molecule COVID-19 medicines: Molnupiravir, nirmatrelvir, and remdesivir.Fundam Clin Pharmacol. 2023 Aug;37(4):726-738. doi: 10.1111/fcp.12889. Epub 2023 Mar 25. Fundam Clin Pharmacol. 2023. PMID: 36931725 Free PMC article. Review.
-
A novel property of hexokinase inhibition by Favipiravir and proposed advantages over Molnupiravir and 2 Deoxy D glucose in treating COVID-19.Biotechnol Lett. 2022 Jul;44(7):831-843. doi: 10.1007/s10529-022-03259-6. Epub 2022 May 24. Biotechnol Lett. 2022. PMID: 35608787 Free PMC article.
Cited by
-
Blood filtering system for COVID-19 management: novel modality of the cytokine storm therapeutics.Front Immunol. 2023 Apr 21;14:1064459. doi: 10.3389/fimmu.2023.1064459. eCollection 2023. Front Immunol. 2023. PMID: 37153613 Free PMC article. Review.
References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 23 September 2022)]. Available online: https://covid19.who.int.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

