Infrared Spectroscopy as a Potential Diagnostic Tool for Medulloblastoma
- PMID: 36903631
- PMCID: PMC10005236
- DOI: 10.3390/molecules28052390
Infrared Spectroscopy as a Potential Diagnostic Tool for Medulloblastoma
Abstract
Introduction: Medulloblastoma (MB) is the most common malignant tumor of the central nervous system in childhood. FTIR spectroscopy provides a holistic view of the chemical composition of biological samples, including the detection of molecules such as nucleic acids, proteins, and lipids. This study evaluated the applicability of FTIR spectroscopy as a potential diagnostic tool for MB.
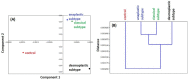
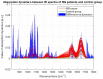
Materials and methods: FTIR spectra of MB samples from 40 children (boys/girls: 31/9; age: median 7.8 years, range 1.5-21.5 years) treated in the Oncology Department of the Children's Memorial Health Institute in Warsaw between 2010 and 2019 were analyzed. The control group consisted of normal brain tissue taken from four children diagnosed with causes other than cancer. Formalin-fixed and paraffin-embedded tissues were sectioned and used for FTIR spectroscopic analysis. The sections were examined in the mid-infrared range (800-3500 cm-1) by ATR-FTIR. Spectra were analysed using a combination of principal component analysis, hierarchical cluster analysis, and absorbance dynamics.
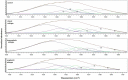
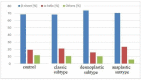
Results: FTIR spectra in MB were significantly different from those of normal brain tissue. The most significant differences related to the range of nucleic acids and proteins in the region 800-1800 cm-1. Some major differences were also revealed in the quantification of protein conformations (α-helices, β-sheets, and others) in the amide I band, as well as in the absorbance dynamics in the 1714-1716 cm-1 range (nucleic acids). It was not, however, possible to clearly distinguish between the various histological subtypes of MB using FTIR spectroscopy.
Conclusions: MB and normal brain tissue can be distinguished from one another to some extent using FTIR spectroscopy. As a result, it may be used as a further tool to hasten and enhance histological diagnosis.
Keywords: FTIR spectroscopy; Fourier transform infrared spectroscopy; MB; medulloblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

