Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.)
- PMID: 36903635
- PMCID: PMC10005433
- DOI: 10.3390/molecules28052386
Bioactive Molecules Derived from Plants in Managing Dengue Vector Aedes aegypti (Linn.)
Abstract
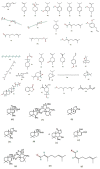
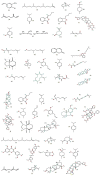
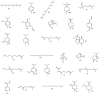
Mosquitoes are the potential vectors of several viral diseases such as filariasis, malaria, dengue, yellow fever, Zika fever and encephalitis in humans as well as other species. Dengue, the most common mosquito-borne disease in humans caused by the dengue virus is transmitted by the vector Ae. aegypti. Fever, chills, nausea and neurological disorders are the frequent symptoms of Zika and dengue. Thanks to various anthropogenic activities such as deforestation, industrialized farming and poor drainage facilities there has been a significant rise in mosquitoes and vector-borne diseases. Control measures such as the destruction of mosquito breeding places, a reduction in global warming, as well as the use of natural and chemical repellents, mainly DEET, picaridin, temephos and IR-3535 have proven to be effective in many instances. Although potent, these chemicals cause swelling, rashes, and eye irritation in adults and children, and are also toxic to the skin and nervous system. Due to their shorter protection period and harmful nature towards non-target organisms, the use of chemical repellents is greatly reduced, and more research and development is taking place in the field of plant-derived repellents, which are found to be selective, biodegradable and harmless to non-target species. Many tribal and rural communities across the world have been using plant-based extracts since ancient times for various traditional and medical purposes, and to ward off mosquitoes and various other insects. In this regard, new species of plants are being identified through ethnobotanical surveys and tested for their repellency against Ae. aegypti. This review aims to provide insight into many such plant extracts, essential oils and their metabolites, which have been tested for their mosquitocidal activity against different life cycle forms of Ae. Aegypti, as well as for their efficacy in controlling mosquitoes.
Keywords: Aedes aegypti; adulticidal; larvicidal; metabolites; non-target toxicity; ovicidal; oviposition deterrent; plant crude extracts; pupicidal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


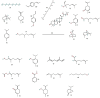
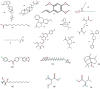
References
-
- Edwin E.-S., Vasantha-Srinivasan P., Senthil-Nathan S., Thanigaivel A., Ponsankar A., Pradeepa V., Selin-Rani S., Kalaivani K., Hunter W.B., Abdel-Megeed A., et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales: Acanthaceae) against the primary dengue vector Aedes aegypti (Diptera: Culicidae) Acta Trop. 2016;163:167–178. doi: 10.1016/j.actatropica.2016.07.009. - DOI - PubMed
-
- Chandra G., Bhattacharjee I., Chatterjee S.N., Ghosh A. Mosquito control by larvivorous fish. Indian J. Med. Res. 2008;127:13–27. - PubMed
-
- World Health Organisation “World Health Day 2014: Preventing Vector-Borne Diseases”. [(accessed on 16 May 2022)]. Available online: https://www.who.int/news/item/02-04-2014-world-health-day-2014-preventin....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

