Bruton's Tyrosine Kinase Inhibitors (BTKIs): Review of Preclinical Studies and Evaluation of Clinical Trials
- PMID: 36903645
- PMCID: PMC10005125
- DOI: 10.3390/molecules28052400
Bruton's Tyrosine Kinase Inhibitors (BTKIs): Review of Preclinical Studies and Evaluation of Clinical Trials
Abstract
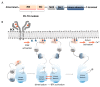
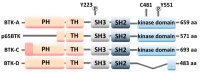
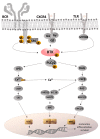
In the last few decades, there has been a growing interest in Bruton's tyrosine kinase (BTK) and the compounds that target it. BTK is a downstream mediator of the B-cell receptor (BCR) signaling pathway and affects B-cell proliferation and differentiation. Evidence demonstrating the expression of BTK on the majority of hematological cells has led to the hypothesis that BTK inhibitors (BTKIs) such as ibrutinib can be an effective treatment for leukemias and lymphomas. However, a growing body of experimental and clinical data has demonstrated the significance of BTK, not just in B-cell malignancies, but also in solid tumors, such as breast, ovarian, colorectal, and prostate cancers. In addition, enhanced BTK activity is correlated with autoimmune disease. This gave rise to the hypothesis that BTK inhibitors can be beneficial in the therapy of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), Sjögren's syndrome (SS), allergies, and asthma. In this review article, we summarize the most recent findings regarding this kinase as well as the most advanced BTK inhibitors that have been developed to date and their clinical applications mainly in cancer and chronic inflammatory disease patients.
Keywords: Bruton’s tyrosine kinase; Bruton’s tyrosine kinase inhibitors; autoimmune disease; cancer; solid tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

