Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation
- PMID: 36903912
- PMCID: PMC10004780
- DOI: 10.3390/plants12051051
Design of an Herbal Preparation Composed by a Combination of Ruscus aculeatus L. and Vitis vinifera L. Extracts, Magnolol and Diosmetin to Address Chronic Venous Diseases through an Anti-Inflammatory Effect and AP-1 Modulation
Abstract
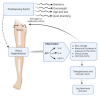
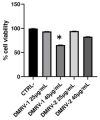
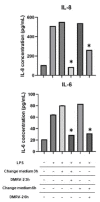
Chronic venous disease (CVD) is an often underestimated inflammatory pathological condition that can have a serious impact on quality of life. Many therapies have been proposed to deal with CVD, but unfortunately the symptoms recur with increasing frequency and intensity as soon as treatments are stopped. Previous studies have shown that the common inflammatory transcription factor AP-1 (activator protein-1) and nuclear factor kappa-activated B-cell light chain enhancer (NF-kB) play key roles in the initiation and progression of this vascular dysfunction. The aim of this research was to develop a herbal product that acts simultaneously on different aspects of CVD-related inflammation. Based on the evidence that several natural components of plant origin are used to treat venous insufficiency and that magnolol has been suggested as a putative modulator of AP-1, two herbal preparations based on Ruscus aculeatus root extracts, and Vitis vinifera seed extracts, as well as diosmetin and magnolol, were established. A preliminary MTT-based evaluation of the possible cytotoxic effects of these preparations led to the selection of one of them, named DMRV-2, for further investigation. First, the anti-inflammatory efficacy of DMRV-2 was demonstrated by monitoring its ability to reduce cytokine secretion from endothelial cells subjected to LPS-induced inflammation. Furthermore, using a real-time PCR-based protocol, the effect of DMRV-2 on AP-1 expression and activity was also evaluated; the results obtained demonstrated that the incubation of the endothelial cells with this preparation almost completely nullified the effects exerted by the treatment with LPS on AP-1. Similar results were also obtained for NF-kB, whose activation was evaluated by monitoring its distribution between the cytosol and the nucleus of endothelial cells after the different treatments.
Keywords: AP-1; MCP-1; Ruscus aculeatus L.; Vitis vinifera L.; anti-inflammatory effects; diosmetin; magnolol; transcriptional factors; venous disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Ruscus aculeatus (butcher's broom) as a potential treatment for orthostatic hypotension, with a case report.J Altern Complement Med. 2000 Dec;6(6):539-49. doi: 10.1089/acm.2000.6.539. J Altern Complement Med. 2000. PMID: 11152059
-
Efficacy and safety of a Butcher's broom preparation (Ruscus aculeatus L. extract) compared to placebo in patients suffering from chronic venous insufficiency.Arzneimittelforschung. 2002;52(4):243-50. doi: 10.1055/s-0031-1299887. Arzneimittelforschung. 2002. PMID: 12040966 Clinical Trial.
-
The effects of Vitis vinifera L. phenolic compounds on a blood-brain barrier culture model: Expression of leptin receptors and protection against cytokine-induced damage.J Ethnopharmacol. 2020 Jan 30;247:112253. doi: 10.1016/j.jep.2019.112253. Epub 2019 Sep 25. J Ethnopharmacol. 2020. PMID: 31562952
-
The place of Ruscus extract, hesperidin methyl chalcone, and vitamin C in the management of chronic venous disease.Int Angiol. 2017 Feb;36(1):31-41. doi: 10.23736/S0392-9590.16.03788-3. Int Angiol. 2017. PMID: 28124877 Review.
-
Combination of Ruscus aculeatus extract, hesperidin methyl chalcone and ascorbic acid: a comprehensive review of their pharmacological and clinical effects and of the pathophysiology of chronic venous disease.Int Angiol. 2016 Apr;35(2):111-6. Int Angiol. 2016. PMID: 26928296 Review.
Cited by
-
Physicochemical Investigations on Samples Composed of a Mixture of Plant Extracts and Biopolymers in the Broad Context of Further Pharmaceutical Development.Polymers (Basel). 2025 May 28;17(11):1499. doi: 10.3390/polym17111499. Polymers (Basel). 2025. PMID: 40508741 Free PMC article.
-
Bioactive Compounds in Citrus Species with Potential for the Treatment of Chronic Venous Disease: A Review.Curr Pharm Des. 2024;30(36):2835-2849. doi: 10.2174/0113816128314974240724045220. Curr Pharm Des. 2024. PMID: 39108121 Review.
-
In vitro and in vivo assessment of diosmetin-loaded lactoferrin-modified liposomes for brain delivery in intracerebral hemorrhage therapy.Drug Deliv Transl Res. 2025 Oct;15(10):3664-3678. doi: 10.1007/s13346-025-01826-8. Epub 2025 Mar 15. Drug Deliv Transl Res. 2025. PMID: 40089650
References
-
- World Health Organization . Global Status Report on Noncommunicable Diseases 2010. World Health Organization; Geneva, Switzerland: 2011.
-
- World Health Organization . Report of the WHO Informal Working Group on Cystic and Alveolar Echninococcosis Surveillance, Prevention and Control, with the Participation of the Food and Agriculture Organization of the United Nations and the World Organisation for Animal Health, 22–23 June 2011. Department of Control of Neglected Tropical Diseases, WHO; Geneva, Switzerland: 2011.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

