Diversity, Distribution, Systematics and Conservation Status of Podocarpaceae
- PMID: 36904033
- PMCID: PMC10005643
- DOI: 10.3390/plants12051171
Diversity, Distribution, Systematics and Conservation Status of Podocarpaceae
Abstract
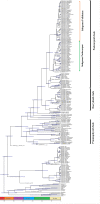
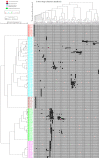
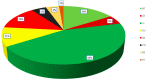
Among conifer families, Podocarpaceae is the second largest, with amazing diversity and functional traits, and it is the dominant Southern Hemisphere conifer family. However, comprehensive studies on diversity, distribution, systematic and ecophysiological aspects of the Podocarpaceae are sparse. We aim to outline and evaluate the current and past diversity, distribution, systematics, ecophysiological adaptations, endemism, and conservation status of podocarps. We analyzed data on the diversity and distribution of living and extinct macrofossil taxa and combined it with genetic data to reconstruct an updated phylogeny and understand historical biogeography. Podocarpaceae today contains 20 genera and approximately 219 taxa (201 species, 2 subspecies, 14 varieties and 2 hybrids) placed in three clades, plus a paraphyletic group/grade of four distinct genera. Macrofossil records show the presence of more than 100 podocarp taxa globally, dominantly from the Eocene-Miocene. Australasia (New Caledonia, Tasmania, New Zealand, and Malesia) is the hotspot of living podocarps diversity. Podocarps also show remarkable adaptations from broad to scale leaves, fleshy seed cones, animal dispersal, shrubs to large trees, from lowland to alpine regions and rheophyte to a parasite (including the only parasitic gymnosperm-Parasitaxus) and a complex pattern of seed and leaf functional trait evolution.
Keywords: IUCN red list; climate change; conifers; conservation; fossils; historical biogeography; paleo-endemism; phylogenetics; physiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Owens J.N., Takaso T., Runions C.J. Pollination in conifers. Trends Plant Sci. 1998;3:479–485. doi: 10.1016/S1360-1385(98)01337-5. - DOI
-
- Conway S. Beyond pine cones: An introduction to gymnosperms. Arnoldia. 2013;70:2–14.
-
- Farjon A. The Kew review: Conifers of the world. Kew Bull. 2018;73:1–16. doi: 10.1007/s12225-018-9738-5. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

