Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration
- PMID: 36904089
- PMCID: PMC10005374
- DOI: 10.3390/nu15051090
Biomimetic Nanozymes Suppressed Ferroptosis to Ameliorate Doxorubicin-Induced Cardiotoxicity via Synergetic Effect of Antioxidant Stress and GPX4 Restoration
Abstract
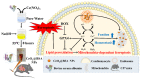
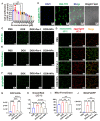
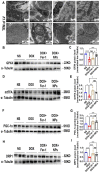
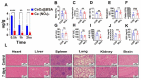
Mitochondria-dependent ferroptosis plays an important role in the pathogenesis of doxorubicin (DOX)-induced cardiotoxicity (DIC), which remains a clinical challenge due to the lack of effective interventions. Cerium oxide (CeO2), a representative nanozyme, has attracted much attention because of its antioxidant properties. This study evaluated CeO2-based nanozymes for the prevention and treatment of DIC in vitro and in vivo by adding nanoparticles (NPs), which were synthesized by biomineralization, to the culture or giving them to the mice, and the ferroptosis-specific inhibitor ferrostatin-1 (Fer-1) was used as control. The prepared NPs exhibited an excellent antioxidant response and glutathione peroxidase 4 (GPX4)-depended bioregulation, with the additional merits of bio-clearance and long retention in the heart. The experiments showed that NP treatment could significantly reverse myocardial structural and electrical remodeling, and reduce myocardial necrosis. These cardioprotective therapeutic effects were associated with their ability to alleviate oxidative stress, mitochondrial lipid peroxidation, and mitochondrial membrane potential damage, with a superior efficiency to the Fer-1. The study also found that the NPs significantly restored the expression of GPX4 and mitochondrial-associated proteins, thereby restoring mitochondria-dependent ferroptosis. Therefore, the study provides some insights into the role of ferroptosis in DIC. It also shows that CeO2-based nanozymes could be a promising prevention and treatment candidate as a novel cardiomyocyte ferroptosis protector to mitigate DIC and improve prognosis and quality of life in cancer patients.
Keywords: biomineralization; doxorubicin-induced cardiomyopathy; ferroptosis; mitochondria; nanozyme; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lipshultz S., Adams M., Colan S., Constine L., Herman E., Hsu D., Hudson M., Kremer L., Landy D., Miller T., et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. - DOI - PubMed
-
- Nabhan C., Byrtek M., Rai A., Dawson K., Zhou X., Link B., Friedberg J., Zelenetz A., Maurer M., Cerhan J., et al. Disease characteristics, treatment patterns, prognosis, outcomes and lymphoma-related mortality in elderly follicular lymphoma in the United States. Br. J. Haematol. 2015;170:85–95. doi: 10.1111/bjh.13399. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2021YJSB278/Graduate Scientific Research Innovation Project of Tianjin Municipal Education Commission
- 81970270/National Natural Science Foundation of China
- 82170327/National Natural Science Foundation of China
- 91959114/National Natural Science Foundation of China
- 81872106/National Natural Science Foundation of China
- 20JCZDJC00340/Natural Science Foundation of Tianjin
- 19JCZDJC33900/Natural Science Foundation of Tianjin
- 20JCJQJC00270/Natural Science Foundation of Tianjin
- TJYXZDXK-029A/Tianjin Key Medical Discipline (Specialty) Construction Project
- TJYXZDXK-070C/Tianjin Key Medical Discipline (Specialty) Construction Project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

