Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants?
- PMID: 36904230
- PMCID: PMC10004767
- DOI: 10.3390/nu15051231
Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants?
Abstract
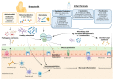
The first objective of infant formulas is to ensure the healthy growth of neonates and infants, as the sole complete food source during the first months of life when a child cannot be breastfed. Beyond this nutritional aspect, infant nutrition companies also try to mimic breast milk in its unique immuno-modulating properties. Numerous studies have demonstrated that the intestinal microbiota under the influence of diet shapes the maturation of the immune system and influences the risk of atopic diseases in infants. A new challenge for dairy industries is, therefore, to develop infant formulas inducing the maturation of immunity and the microbiota that can be observed in breastfed delivered vaginally, representing reference infants. Streptococcus thermophilus, Lactobacillus reuteri DSM 17938, Bifidobacterium breve (BC50), Bifidobacterium lactis Bb12, Lactobacillus fermentum (CECT5716), and Lactobacillus rhamnosus GG (LGG) are some of the probiotics added to infant formula, according to a literature review of the past 10 years. The most frequently used prebiotics in published clinical trials are fructo-oligosaccharides (FOSs), galacto-oligosaccharides (GOSs), and human milk oligosaccharides (HMOs). This review sums up the expected benefits and effects for infants of pre-, pro-, syn-, and postbiotics added to infant formula regarding the microbiota, immunity, and allergies.
Keywords: atopy; immunity; infant formula; microbiota; postbiotic; prebiotic; probiotic; synbiotic.
Conflict of interest statement
AL received an honorarium and has been involved as a consultant for Nestlé, Nutricia, Mead Johnson/Reckitt, and Sodilac. PT received an honorarium and has been involved as a consultant for Carrefour, Danone, Mead Johnson/Reckitt, Nestlé, Novalac/Ménarini, SILL, and Sodilac. KAP’s lab received grants from Nestlé and Mead Johnson/Reckitt for collaborative research work or service offers. MT declares no conflicts of interest. The authors declare that the conflicts of interest had no influence on the present review.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

