Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering
- PMID: 36904322
- PMCID: PMC10006987
- DOI: 10.3390/polym15051079
Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering
Abstract
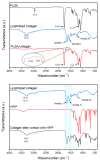
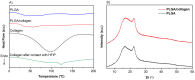
The development of scaffolding obtained by electrospinning is widely used in tissue engineering due to porous and fibrous structures that can mimic the extracellular matrix. In this study, poly (lactic-co-glycolic acid) (PLGA)/collagen fibers were fabricated by electrospinning method and then evaluated in the cell adhesion and viability of human cervical carcinoma HeLa and NIH-3T3 fibroblast for potential application in tissue regeneration. Additionally, collagen release was assessed in NIH-3T3 fibroblasts. The fibrillar morphology of PLGA/collagen fibers was verified by scanning electron microscopy. The fiber diameter decreased in the fibers (PLGA/collagen) up to 0.6 µm. FT-IR spectroscopy and thermal analysis confirmed that both the electrospinning process and the blend with PLGA give structural stability to collagen. Incorporating collagen in the PLGA matrix promotes an increase in the material's rigidity, showing an increase in the elastic modulus (38%) and tensile strength (70%) compared to pure PLGA. PLGA and PLGA/collagen fibers were found to provide a suitable environment for the adhesion and growth of HeLa and NIH-3T3 cell lines as well as stimulate collagen release. We conclude that these scaffolds could be very effective as biocompatible materials for extracellular matrix regeneration, suggesting their potential applications in tissue bioengineering.
Keywords: HeLa cells; NIH-3T3 fibroblasts; PLGA; collagen; electrospun fibers; scaffolds; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Rahmati M., Mills D.K., Urbanska A.M., Saeb M.R., Venugopal J.R., Ramakrishna S., Mozafari M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021;117:100721. doi: 10.1016/j.pmatsci.2020.100721. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

