Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials
- PMID: 36904500
- PMCID: PMC10007465
- DOI: 10.3390/polym15051259
Imidazolium Salts for Candida spp. Antibiofilm High-Density Polyethylene-Based Biomaterials
Abstract
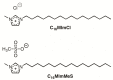
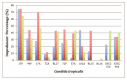
The species of Candida present good capability to form fungal biofilms on polymeric surfaces and are related to several human diseases since many of the employed medical devices are designed using polymers, especially high-density polyethylene (HDPE). Herein, HDPE films containing 0; 0.125; 0.250 or 0.500 wt% of 1-hexadecyl-3-methylimidazolium chloride (C16MImCl) or its analog 1-hexadecyl-3-methylimidazolium methanesulfonate (C16MImMeS) were obtained by melt blending and posteriorly mechanically pressurized into films. This approach resulted in more flexible and less brittle films, which impeded the Candida albicans, C. parapsilosis, and C. tropicalis biofilm formation on their surfaces. The employed imidazolium salt (IS) concentrations did not present any significant cytotoxic effect, and the good cell adhesion/proliferation of human mesenchymal stem cells on the HDPE-IS films indicated good biocompatibility. These outcomes combined with the absence of microscopic lesions in pig skin after contact with HDPE-IS films demonstrated their potential as biomaterials for the development of effective medical device tools that reduce the risk of fungal infections.
Keywords: biocompatibility; histopathological evaluation; human mesenchymal stem cells; ionic liquid; melt blending.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

