A New Generation of OPM for High Dynamic and Large Bandwidth MEG: The 4He OPMs-First Applications in Healthy Volunteers
- PMID: 36905007
- PMCID: PMC10006929
- DOI: 10.3390/s23052801
A New Generation of OPM for High Dynamic and Large Bandwidth MEG: The 4He OPMs-First Applications in Healthy Volunteers
Abstract
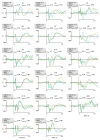
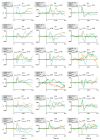
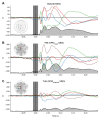
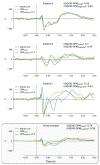
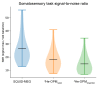
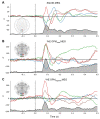
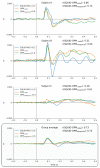
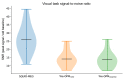
MagnetoEncephaloGraphy (MEG) provides a measure of electrical activity in the brain at a millisecond time scale. From these signals, one can non-invasively derive the dynamics of brain activity. Conventional MEG systems (SQUID-MEG) use very low temperatures to achieve the necessary sensitivity. This leads to severe experimental and economical limitations. A new generation of MEG sensors is emerging: the optically pumped magnetometers (OPM). In OPM, an atomic gas enclosed in a glass cell is traversed by a laser beam whose modulation depends on the local magnetic field. MAG4Health is developing OPMs using Helium gas (4He-OPM). They operate at room temperature with a large dynamic range and a large frequency bandwidth and output natively a 3D vectorial measure of the magnetic field. In this study, five 4He-OPMs were compared to a classical SQUID-MEG system in a group of 18 volunteers to evaluate their experimental performances. Considering that the 4He-OPMs operate at real room temperature and can be placed directly on the head, our assumption was that 4He-OPMs would provide a reliable recording of physiological magnetic brain activity. Indeed, the results showed that the 4He-OPMs showed very similar results to the classical SQUID-MEG system by taking advantage of a shorter distance to the brain, despite having a lower sensitivity.
Keywords: MEG; OPM; SQUID; atomic magnetometer; brain activity; helium OPM; neuroimaging.
Conflict of interest statement
The authors declare the following competing interests: M.L.P., A.P.-L. and E.L. hold founding equity in Mag4Health SAS, a French start-up company that is developing and commercializing MEG systems based on He-OPM technology. R.R. and S.M. are employees of Mag4Health SAS. Mag4Health SAS provided technical support to the data acquisition. For the recordings performed until 1 February 2022, S.M., A.P.-L. and E.L. were still employees of CEA LETI.
Figures


References
-
- Budker D., Romalis M.V. Optical Magnetometry. Nat. Phys. 2006;3:277. doi: 10.1038/nphys566. - DOI
-
- Boto E., Meyer S.S., Shah V., Alem O., Knappe S., Kruger P., Fromhold T.M., Lim M., Glover P.M., Morris P.G., et al. A New Generation of Magnetoencephalography: Room Temperature Measurements Using Optically-Pumped Magnetometers. Neuroimage. 2017;149:404–414. doi: 10.1016/j.neuroimage.2017.01.034. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

