Structure, regulation, and physiological functions of NADPH oxidase 5 (NOX5)
- PMID: 36905456
- PMCID: PMC10300161
- DOI: 10.1007/s13105-023-00955-3
Structure, regulation, and physiological functions of NADPH oxidase 5 (NOX5)
Abstract
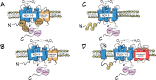
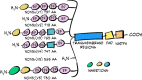
NOX5 is the last member of the NADPH oxidase (NOXs) family to be identified and presents some specific characteristics differing from the rest of the NOXs. It contains four Ca2+ binding domains at the N-terminus and its activity is regulated by the intracellular concentration of Ca2+. NOX5 generates superoxide (O2•-) using NADPH as a substrate, and it modulates functions related to processes in which reactive oxygen species (ROS) are involved. Those functions appear to be detrimental or beneficial depending on the level of ROS produced. For example, the increase in NOX5 activity is related to the development of various oxidative stress-related pathologies such as cancer, cardiovascular, and renal diseases. In this context, pancreatic expression of NOX5 can negatively alter insulin action in high-fat diet-fed transgenic mice. This is consistent with the idea that the expression of NOX5 tends to increase in response to a stimulus or a stressful situation, generally causing a worsening of the pathology. On the other hand, it has also been suggested that it might have a positive role in preparing the body for metabolic stress, for example, by inducing a protective adipose tissue adaptation to the excess of nutrients supplied by a high-fat diet. In this line, its endothelial overexpression can delay lipid accumulation and insulin resistance development in obese transgenic mice by inducing the secretion of IL-6 followed by the expression of thermogenic and lipolytic genes. However, as NOX5 gene is not present in rodents and human NOX5 protein has not been crystallized, its function is still poorly characterized and further extensive research is required.
Keywords: Inflammation; Insulin resistance; NADPH oxidase; NOX; Obesity; Oxidative stress.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ahmarani L, Avedanian L, Al-Khoury J, Perreault C, Jacques D, Bkaily G. Whole-cell and nuclear NADPH oxidases levels and distribution in human endocardial endothelial, vascular smooth muscle, and vascular endothelial cells. Can J Physiol Pharmacol. 2013;91:71–79. doi: 10.1139/cjpp-2012-0265. - DOI - PubMed
-
- Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

