Prognostic value of cell-free DNA in cerebrospinal fluid from lung cancer patients with brain metastases during radiotherapy
- PMID: 36906568
- PMCID: PMC10007729
- DOI: 10.1186/s13014-023-02239-y
Prognostic value of cell-free DNA in cerebrospinal fluid from lung cancer patients with brain metastases during radiotherapy
Abstract
Background: During the last decades, radiotherapy (RT) for non-small cell lung cancer (NSCLC) with brain metastases (BM) has been developed. However, the lack of predictive biomarkers for therapeutic responses has limited the precision treatment in NSCLC-BM.
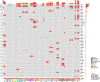
Patients and methods: In order to find the predictive biomarkers for RT, we investigated the influence of RT on the cell-free DNA (cfDNA) from cerebrospinal fluid (CSF) and the frequency of T cell subsets of NSCLC patients with BM. A total of 19 patients diagnosed as NSCLC with BM were enrolled. The CSF from 19 patients and matched plasma samples from 11 patients were collected before RT, during RT, and after RT. The cfDNA from CSF and plasma were extracted, and the cerebrospinal fluid tumor mutation burden (cTMB) was calculated after through next-generation sequencing. The frequency of T cell subsets in peripheral blood was using flow cytometry.
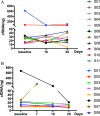
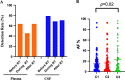
Results: The detection rate of cfDNA was higher in CSF compared to plasma in the matched samples. The mutation abundance of cfDNA in CSF was decreased after RT. However, no significant difference was observed in cTMB before and after RT. Although the median intracranial progression-free survival (iPFS) has not yet been reached in patients with decreased or undetectable cTMB, there was a trend that these patients possessed longer iPFS compared to those with stable or increased cTMB (HR 0.28, 95% CI 0.07-1.18, P = 0.067). The proportion of CD4+T cells in peripheral blood was decreased after RT.
Conclusion: Our study indicates that cTMB can serve as a prognostic biomarker in NSCLC patients with BMs.
Keywords: Brain metastases; Cell-free DNA; NSCLC; Radiotherapy; cTMB.
© 2023. The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy.Ann Oncol. 2018 Apr 1;29(4):945-952. doi: 10.1093/annonc/mdy009. Ann Oncol. 2018. PMID: 29346604
-
Genetic profiling of circulating cell-free DNA from cerebrospinal fluid and paired plasma in ALK-positive lung cancer with brain metastases.Thorac Cancer. 2023 Jul;14(19):1883-1893. doi: 10.1111/1759-7714.14936. Epub 2023 Jun 7. Thorac Cancer. 2023. PMID: 37287428 Free PMC article.
-
Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples.Thorac Cancer. 2020 Mar;11(3):588-593. doi: 10.1111/1759-7714.13300. Epub 2020 Jan 13. Thorac Cancer. 2020. PMID: 31944608 Free PMC article.
-
Response rate of patients with baseline brain metastases from recently diagnosed non-small cell lung cancer receiving radiotherapy according to EGFR, ALK and KRAS mutation status.Thorac Cancer. 2020 Apr;11(4):1026-1037. doi: 10.1111/1759-7714.13359. Epub 2020 Feb 19. Thorac Cancer. 2020. PMID: 32072746 Free PMC article.
-
Unique genomic profiles obtained from cerebrospinal fluid cell-free DNA of non-small cell lung cancer patients with leptomeningeal metastases.Cancer Biol Ther. 2019;20(4):562-570. doi: 10.1080/15384047.2018.1538614. Epub 2018 Nov 5. Cancer Biol Ther. 2019. PMID: 30395779 Free PMC article.
Cited by
-
Metastatic brain tumors: from development to cutting-edge treatment.MedComm (2020). 2024 Dec 20;6(1):e70020. doi: 10.1002/mco2.70020. eCollection 2025 Jan. MedComm (2020). 2024. PMID: 39712454 Free PMC article. Review.
References
-
- Dempke WC, Edvardsen K, Lu S, et al. Brain metastases in NSCLC - are TKIs changing the treatment strategy? Anticancer Res. 2015;35:5797–5806. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

