[18F]F-DED PET imaging of reactive astrogliosis in neurodegenerative diseases: preclinical proof of concept and first-in-human data
- PMID: 36906584
- PMCID: PMC10007845
- DOI: 10.1186/s12974-023-02749-2
[18F]F-DED PET imaging of reactive astrogliosis in neurodegenerative diseases: preclinical proof of concept and first-in-human data
Abstract
Objectives: Reactive gliosis is a common pathological hallmark of CNS pathology resulting from neurodegeneration and neuroinflammation. In this study we investigate the capability of a novel monoamine oxidase B (MAO-B) PET ligand to monitor reactive astrogliosis in a transgenic mouse model of Alzheimer`s disease (AD). Furthermore, we performed a pilot study in patients with a range of neurodegenerative and neuroinflammatory conditions.
Methods: A cross-sectional cohort of 24 transgenic (PS2APP) and 25 wild-type mice (age range: 4.3-21.0 months) underwent 60 min dynamic [18F]fluorodeprenyl-D2 ([18F]F-DED), static 18 kDa translocator protein (TSPO, [18F]GE-180) and β-amyloid ([18F]florbetaben) PET imaging. Quantification was performed via image derived input function (IDIF, cardiac input), simplified non-invasive reference tissue modelling (SRTM2, DVR) and late-phase standardized uptake value ratios (SUVr). Immunohistochemical (IHC) analyses of glial fibrillary acidic protein (GFAP) and MAO-B were performed to validate PET imaging by gold standard assessments. Patients belonging to the Alzheimer's disease continuum (AD, n = 2), Parkinson's disease (PD, n = 2), multiple system atrophy (MSA, n = 2), autoimmune encephalitis (n = 1), oligodendroglioma (n = 1) and one healthy control underwent 60 min dynamic [18F]F-DED PET and the data were analyzed using equivalent quantification strategies.
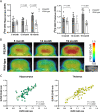
Results: We selected the cerebellum as a pseudo-reference region based on the immunohistochemical comparison of age-matched PS2APP and WT mice. Subsequent PET imaging revealed that PS2APP mice showed elevated hippocampal and thalamic [18F]F-DED DVR when compared to age-matched WT mice at 5 months (thalamus: + 4.3%; p = 0.048), 13 months (hippocampus: + 7.6%, p = 0.022) and 19 months (hippocampus: + 12.3%, p < 0.0001; thalamus: + 15.2%, p < 0.0001). Specific [18F]F-DED DVR increases of PS2APP mice occurred earlier when compared to signal alterations in TSPO and β-amyloid PET and [18F]F-DED DVR correlated with quantitative immunohistochemistry (hippocampus: R = 0.720, p < 0.001; thalamus: R = 0.727, p = 0.002). Preliminary experience in patients showed [18F]F-DED VT and SUVr patterns, matching the expected topology of reactive astrogliosis in neurodegenerative (MSA) and neuroinflammatory conditions, whereas the patient with oligodendroglioma and the healthy control indicated [18F]F-DED binding following the known physiological MAO-B expression in brain.
Conclusions: [18F]F-DED PET imaging is a promising approach to assess reactive astrogliosis in AD mouse models and patients with neurological diseases.
Keywords: Astrocytes; Deprenyl; MAO-B; PET.
© 2023. The Author(s).
Conflict of interest statement
NK, AM and AWS are employees of Life Molecular Imaging. MB received research funding from Life Molecular Imaging. No other potential competing interest relevant to this article exist.
Figures




References
-
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169:1276–1290.e1217. doi: 10.1016/j.cell.2017.05.018. - DOI - PubMed
-
- Rostami J, Mothes T, Kolahdouzan M, Eriksson O, Moslem M, Bergström J, Ingelsson M, O'Callaghan P, Healy LM, Falk A, Erlandsson A. Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J Neuroinflammation. 2021;18:124. doi: 10.1186/s12974-021-02158-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

