SMAD2/3 signaling in the uterine epithelium controls endometrial cell homeostasis and regeneration
- PMID: 36906706
- PMCID: PMC10008566
- DOI: 10.1038/s42003-023-04619-2
SMAD2/3 signaling in the uterine epithelium controls endometrial cell homeostasis and regeneration
Abstract
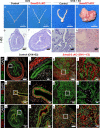
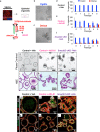
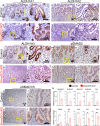
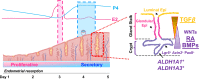
The regenerative potential of the endometrium is attributed to endometrial stem cells; however, the signaling pathways controlling its regenerative potential remain obscure. In this study, genetic mouse models and endometrial organoids are used to demonstrate that SMAD2/3 signaling controls endometrial regeneration and differentiation. Mice with conditional deletion of SMAD2/3 in the uterine epithelium using Lactoferrin-iCre develop endometrial hyperplasia at 12-weeks and metastatic uterine tumors by 9-months of age. Mechanistic studies in endometrial organoids determine that genetic or pharmacological inhibition of SMAD2/3 signaling disrupts organoid morphology, increases the glandular and secretory cell markers, FOXA2 and MUC1, and alters the genome-wide distribution of SMAD4. Transcriptomic profiling of the organoids reveals elevated pathways involved in stem cell regeneration and differentiation such as the bone morphogenetic protein (BMP) and retinoic acid signaling (RA) pathways. Therefore, TGFβ family signaling via SMAD2/3 controls signaling networks which are integral for endometrial cell regeneration and differentiation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

