Pharmacological interventions for persistent postural-perceptual dizziness (PPPD)
- PMID: 36906836
- PMCID: PMC9997546
- DOI: 10.1002/14651858.CD015188.pub2
Pharmacological interventions for persistent postural-perceptual dizziness (PPPD)
Abstract
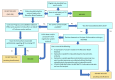
Background: Persistent postural-perceptual dizziness (PPPD) is a chronic balance disorder, which is characterised by subjective unsteadiness or dizziness that is worse on standing and with visual stimulation. The condition was only recently defined and therefore the prevalence is currently unknown. However, it is likely to include a considerable number of people with chronic balance problems. The symptoms can be debilitating and have a profound impact on quality of life. At present, little is known about the optimal way to treat this condition. A variety of medications may be used, as well as other treatments, such as vestibular rehabilitation. OBJECTIVES: To evaluate the benefits and harms of pharmacological interventions for persistent postural-perceptual dizziness (PPPD). SEARCH METHODS: The Cochrane ENT Information Specialist searched the Cochrane ENT Register; Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 21 November 2022.
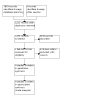
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs in adults with PPPD, which compared selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs) with either placebo or no treatment. We excluded studies that did not use the Bárány Society criteria to diagnose PPPD and studies that followed up participants for less than three months. DATA COLLECTION AND ANALYSIS: We used standard Cochrane methods. Our primary outcomes were: 1) improvement in vestibular symptoms (assessed as a dichotomous outcome - improved or not improved), 2) change in vestibular symptoms (assessed as a continuous outcome, with a score on a numerical scale) and 3) serious adverse events. Our secondary outcomes were: 4) disease-specific health-related quality of life, 5) generic health-related quality of life and 6) other adverse effects. We considered outcomes reported at three time points: 3 to < 6 months, 6 to ≤ 12 months and > 12 months. We planned to use GRADE to assess the certainty of evidence for each outcome. MAIN RESULTS: We identified no studies that met our inclusion criteria.
Authors' conclusions: At present, there is no evidence from placebo-controlled randomised trials regarding pharmacological treatments - specifically SSRIs and SNRIs - for PPPD. Consequently, there is great uncertainty over the use of these treatments for this condition. Further work is needed to establish whether any treatments are effective at improving the symptoms of PPPD, and whether their use is associated with any adverse effects.
배경: 지속성 체위‐지각 어지럼증(PPPD)은 만성 균형 장애로, 서 있을 때나 시각적 자극이 있을 때 더 악화되는 주관적인 불안정 또는 현기증을 특징으로 한다. 이 조건은 최근에야 정의되었으므로 현재 유병률을 알 수 없다. 그러나 만성 균형 문제가 있는 상당수의 사람들이 포함될 가능성이 높다. 증상은 쇠약해지고 삶의 질에 지대한 영향을 미칠 수 있다. 현재 이 상태를 치료하는 최적의 방법에 대해서는 알려진 바가 거의 없다. 전정 재활과 같은 다른 치료뿐만 아니라 다양한 약물이 사용될 수 있다. 목적: 지속성 체위‐지각 어지럼증(PPPD)에 대한 약리학적 중재의 이점과 위해를 평가한다. 검색 전략: Cochrane ENT 정보 전문가가 Cochrane ENT Register, 통제된 시험의 중앙 등록부(CENTRAL); 오비드 메드라인; 오비드 엠베이스; 웹 오브 사이언스; ClinicalTrials.gov; 공개 및 미공개 시험에 대한 ICTRP 및 추가 출처를 검색했다. 검색 날짜는 2022년 11월 21일이었다. 선정 기준: 선택적 세로토닌 재흡수 억제제(SSRI) 또는 세로토닌 및 노르에피네프린 재흡수 억제제(SNRI)를 위약 또는 무치료와 비교한 PPPD 성인을 대상으로 한 무작위 대조 시험(RCT) 및 준 RCT를 포함했다. PPPD를 진단하기 위해 Bárány Society 기준을 사용하지 않은 연구와 3개월 미만 동안 참가자를 추적한 연구는 제외했다. 자료 수집 및 분석: 표준 코크란 방법을 사용했다. 주요 결과는 다음과 같다. 1) 전정 증상의 개선(이분법적 결과로 평가 ‐ 개선 또는 개선되지 않음), 2) 전정 증상의 변화(숫자 척도에서 점수를 사용하여 연속 결과로 평가) 및 3) 심각한 부작용. 이차 결과는 다음과 같다. 4) 질병 특정 건강 관련 삶의 질, 5) 일반적인 건강 관련 삶의 질 및 6) 기타 부작용. 세 가지 시점에서 보고된 결과를 고려했다. 3 ~ 6개월 미만, 6 ~ 12개월 이하 및 > 12개월 초과. 각 결과에 대한 근거의 확실성을 평가하기 위해 GRADE를 사용할 계획이었다. 주요 결과: 포함 기준에 부합하는 연구를 검색하지 못했다. 연구진 결론: 현재 PPPD에 대한 약리학적 치료(특히 SSRI 및 SNRI)에 관한 위약 대조 무작위 시험의 근거는 없다. 결과적으로, 이 상태에 대한 이러한 치료법의 사용에 대해 큰 불확실성이 있다. PPPD의 증상을 개선하는 데 효과적인 치료법이 있는지, 그리고 그 사용이 부작용과 관련이 있는지 여부를 확인하려면 추가 연구가 필요하다.
پیشینه: سرگیجه مداوم وضعیتی‐ادراکی (persistent postural‐perceptual dizziness; PPPD) یک اختلال تعادلی مزمن است، که با بیثباتی سابجکتیو یا سرگیجه مشخص میشود که در حالت ایستادن و با تحریک بینایی بدتر میشود. این وضعیت اخیرا تعریف شده و بنابراین شیوع آن در حال حاضر ناشناخته است. با این حال، احتمالا شامل تعداد قابلتوجهی از افراد با مشکلات تعادلی مزمن میشود. نشانهها میتوانند ناتوان کننده بوده و تاثیر عمیقی بر کیفیت زندگی بیمار داشته باشند. در حال حاضر، اطلاعات کمی در مورد روش مطلوب درمان این وضعیت وجود دارد. ممکن است از انواع داروها، همچنین درمانهای دیگر مانند توانبخشی دهلیزی، استفاده شود. اهداف: ارزیابی مزایا و مضرات مداخلات دارویی در مدیریت بالینی سرگیجه مداوم وضعیتی‐ادراکی (PPPD). روشهای جستوجو: متخصص اطلاعات گروه گوش و حلق و بینی (ENT) در کاکرین برای یافتن کارآزماییهای منتشر شده و منتشر نشده به جستوجو در پایگاه ثبت گروه گوش و حلق و بینی (ENT) در کاکرین؛ پایگاه مرکزی ثبت کارآزماییهای کنترل شده کاکرین (CENTRAL)؛ Ovid MEDLINE؛ Ovid Embase؛ Web of Science؛ ClinicalTrials.gov؛ ICTRP و منابع دیگر پرداخت. تاریخ جستوجو 21 نوامبر 2022 بود. معیارهای انتخاب: کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTها را با حضور بزرگسالان مبتلا به PPPD وارد کردیم، که مهار کنندههای انتخابی بازجذب سروتونین (SSRIs) یا مهار کنندههای بازجذب سروتونین و نوراپینفرین (SNRIs) را با دارونما (placebo) یا عدم درمان مقایسه کردند. مطالعاتی را حذف کردیم که از معیارهای Bárány Society برای تشخیص PPPD استفاده نکرده و مطالعاتی که شرکتکنندگان را کمتر از سه ماه دنبال کردند. گردآوری و تجزیهوتحلیل دادهها: از روشهای استاندارد کاکرین بهره بردیم. پیامدهای اولیه عبارت بودند از: 1) بهبود نشانههای دهلیزی (ارزیابی شده به صورت یک پیامد دو حالتی (dichotomous) ‐ بهبود یافته یا بهبود نیافته)، 2) تغییر در نشانههای دهلیزی (در قالب یک پیامد پیوسته (continuous)، با نمره در یک مقیاس عددی) و 3) عوارض جانبی جدی. پیامدهای ثانویه شامل: 4) کیفیت زندگی مرتبط با سلامت و مرتبط با بیماری خاص، 5) کیفیت زندگی مرتبط با سلامت عمومی و 6) دیگر عوارض جانبی، بودند. پیامدهای گزارش شده را در سه مقطع زمانی در نظر گرفتیم: 3 تا < 6 ماه، 6 تا ≤ 12 ماه و > 12 ماه. از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (Grading of Recommendations Assessment, Development and Evaluation; GRADE) برای ارزیابی قطعیت شواهد برای هر پیامد بهره گرفتیم. نتایج اصلی: هیچ مطالعهای را شناسایی نکردیم که معیارهای ورود را داشته باشد. نتیجهگیریهای نویسندگان: در حال حاضر، هیچ شواهدی از کارآزماییهای تصادفیسازی شده و کنترل شده با دارونما در مورد درمانهای دارویی ‐ بهویژه SSRIها و SNRIها ‐ برای PPPD وجود ندارد. در نتیجه، عدم اطمینان زیادی در مورد استفاده از این درمانها برای این وضعیت وجود دارد. برای تعیین اینکه چه درمانی در بهبود نشانههای PPPD موثر است، و اینکه استفاده از آنها با عوارض جانبی مرتبط است یا خیر، به کار بیشتری نیاز است.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Katie Webster: none known.
Natasha A Harrington‐Benton: Natasha Harrington‐Benton is the Director of the Ménière’s Society, a national charity supporting people with vestibular conditions. The Ménière’s Society supports research in various ways, including distributing surveys and/or providing grant funding for projects studying vestibular conditions. Some of the studies they have previously funded may be included in the review. They do not carry out the research themselves and are not directly involved in projects.
Owen Judd: none known.
Diego Kaski: none known.
Otto R Maarsingh: none known.
Samuel MacKeith: Samuel MacKeith is the Assistant Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Jaydip Ray: none known.
Vincent A Van Vugt: none known.
Martin J Burton: Martin Burton undertook private practice until March 2020 and saw some patients with PPPD. He is the Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Figures
Update of
References
References to studies excluded from this review
Basta 2017 {published data only}
-
- Basta D, Borsellino L, Ernst A. Antivertiginous drug therapy does not hinder the efficacy of individualized vibrotactile neurofeedback training for vestibular rehabilitation - a randomized trial. International Journal of Rehabilitation Research 2017;40(4):333-8. - PubMed
Additional references
Balaban 2002
-
- Balaban C. Neural substrates linking balance control and anxiety. Physiology and Behavior 2002;77(4-5):469-75. - PubMed
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trials in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. - PubMed
EuroQol 1990
-
- EuroQol group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 1990;16(3):199-208. - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Handbook 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Herdman 2011
Jacobsen 1990
-
- Jacobsen GP, Newman CW. The development of the Dizziness Handicap Inventory. Archives of Otolaryngology--Head and Neck Surgery 1990;116(4):424-7. - PubMed
Jacobsen 1998
-
- Jacobsen GP, Calder JH. A screening version of the Dizziness Handicap Inventory. American Journal of Otology 1998;19(6):804-8. - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Popkirov 2018
-
- Popkirov S, Staab JP, Stone J. Persistent postural-perceptual dizziness (PPPD): a common, characteristic and treatable cause of chronic dizziness. Practical Neurology 2018;18(1):5. - PubMed
Sezier 2019
Staab 2017
-
- Staab JP, Eckhardt-Henn A, Horii A, Jacob R, Strupp M, Brandt T, et al. Diagnostic criteria for persistent postural-perceptual dizziness (PPPD): Consensus document of the committee for the Classification of Vestibular Disorders of the Bárány Society. Journal of Vestibular Research 2017;27(4):191-208. [DOI: 10.3233/VES-170622] [PMID: PMID: 29036855] - DOI - PMC - PubMed
Staab 2020
-
- Staab J. Persistent postural-perceptual dizziness. Seminars in Neurology 2020;40:130-7. - PubMed
Tesio 1999
-
- Tesio L, Alpini D, Cesarani A, Perucca L. Short form of the Dizziness Handicap Inventory: construction and validation through Rasch analysis. American Journal of Physical Medicine and Rehabilitation 1999;78(3):233-41. - PubMed
Ware 1992
-
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. - PubMed
Webster 2022a
References to other published versions of this review
Webster 2022b
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous