The effect of GLP-1RA exenatide on idiopathic intracranial hypertension: a randomized clinical trial
- PMID: 36907221
- PMCID: PMC10151178
- DOI: 10.1093/brain/awad003
The effect of GLP-1RA exenatide on idiopathic intracranial hypertension: a randomized clinical trial
Abstract
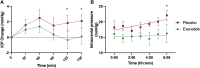
Therapeutics to reduce intracranial pressure are an unmet need. Preclinical data have demonstrated a novel strategy to lower intracranial pressure using glucagon-like peptide-1 (GLP-1) receptor signalling. Here, we translate these findings into patients by conducting a randomized, placebo-controlled, double-blind trial to assess the effect of exenatide, a GLP-1 receptor agonist, on intracranial pressure in idiopathic intracranial hypertension. Telemetric intracranial pressure catheters enabled long-term intracranial pressure monitoring. The trial enrolled adult women with active idiopathic intracranial hypertension (intracranial pressure >25 cmCSF and papilloedema) who receive subcutaneous exenatide or placebo. The three primary outcome measures were intracranial pressure at 2.5 h, 24 h and 12 weeks and alpha set a priori at less than 0.1. Among the 16 women recruited, 15 completed the study (mean age 28 ± 9, body mass index 38.1 ± 6.2 kg/m2, intracranial pressure 30.6 ± 5.1 cmCSF). Exenatide significantly and meaningfully lowered intracranial pressure at 2.5 h -5.7 ± 2.9 cmCSF (P = 0.048); 24 h -6.4 ± 2.9 cmCSF (P = 0.030); and 12 weeks -5.6 ± 3.0 cmCSF (P = 0.058). No serious safety signals were noted. These data provide confidence to proceed to a phase 3 trial in idiopathic intracranial hypertension and highlight the potential to utilize GLP-1 receptor agonist in other conditions characterized by raised intracranial pressure.
Keywords: cerebrospinal fluid; glucagon-like peptide 1; idiopathic intracranial hypertension; intracranial pressure; telemetric monitor.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
J.L.M. was funded by the UK Ministry of Defence for the duration of the study. O.G. reports scientific consultancy fees from Invex therapeutics (2020). A.Y. reports receiving speaker fees from Teva, UK, outside the submitted work. K.B. works for UCB. Professor Mollan reports other Invex Therapeutics, other Heidelberg engineering during the conduct of the study; other from Chugai-Roche Ltd, other from Janssen, other from Allergan, other from Santen, other from Roche, other from Neurodiem, outside the submitted work. Professor Sinclair reports personal fees from Invex therapeutics in her role as Director with stock holdings, during the conduct of the study (since 28.06.2019); other from Allergan, Novartis, Cheisi and Amgen outside the submitted work. All other authors declare no competing interests.
Figures


References
-
- Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002;122:531–544. - PubMed
-
- Alvarez E, Roncero I, Chowen JA, Thorens B, Blázquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66:920–927. - PubMed
-
- Carr RD, Larsen MO, Jelic K, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. Comparative study. J Clin Endocrinol Metab. 2010;95:872–878. - PubMed
-
- Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. Clinical trial. J Clin Endocrinol Metab. 2004;89:3055–3061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

