Sex, racial, and ethnic diversity in clinical trials
- PMID: 36908052
- PMCID: PMC10264921
- DOI: 10.1111/cts.13513
Sex, racial, and ethnic diversity in clinical trials
Abstract
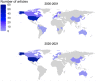
Diverse representation in clinical trials is crucial to understand the efficacy and safety of drugs in minority groups. This review aims to (1) describe research participants' sex, racial, and ethnic diversity in clinical drug trials and (2) describe the sex distribution of researchers conducting the research. We reviewed all clinical drug trials published in the journals "Clinical Pharmacology and Therapeutics" and "Clinical and Translational Science" in 2000-2001 and 2020-2021 and analyzed the research participants' and researchers' demographics. We compared the race of the research participants with the concurrent race diversity of the reference population in the countries where the research was conducted. We identified 281 articles with 17,639 research participants. Approximately one-third of the research participants were women in both 2000-2001 and 2020-2021. The representation from racial minorities of Black and Asian people increased from 2000-2001 to 2020-2021, but Asian and Native American people are still under-represented in clinical drug trials today. The proportion of female authors increased, but female authors still made up less than 40% of the total number of authors in 2020-2021. In conclusion, men are still over-represented in clinical pharmacology research, and some races are still vastly under-represented. Furthermore, although the proportion of female authors increased with time, they are still under-represented as first and last authors.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.D. has given paid lectures for Astellas Pharma. T.S has given paid lectures for Pfizer and Eisai and consulted for Pfizer. All other authors declared no competing interests for this work.
Figures
References
-
- NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research | grants.nih.gov [Internet]. Available at: https://grants.nih.gov/policy/inclusion/women‐and‐minorities/guidelines.htm. Accessed March 15, 2022.
-
- Spoletini I, Vitale C, Malorni W, Rosano GMC. Sex differences in drug effects: interaction with sex hormones in adult life. Handb Exp Pharmacol. 2012;214:91‐105. - PubMed
-
- Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55(2):81‐95. - PubMed
-
- Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health. 2005;14(1):19‐29. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

