Modeling the accuracy of a novel PCR and antibody ELISA for African swine fever virus detection using Bayesian latent class analysis
- PMID: 36908521
- PMCID: PMC9995851
- DOI: 10.3389/fvets.2023.1079918
Modeling the accuracy of a novel PCR and antibody ELISA for African swine fever virus detection using Bayesian latent class analysis
Abstract
Introduction: Diagnostic test evaluation for African swine fever (ASF) in field settings like Vietnam is critical to understanding test application in intended populations for surveillance and control strategies. Bayesian latent class analysis (BLCA) uses the results of multiple imperfect tests applied to an individual of unknown disease status to estimate the diagnostic sensitivity and specificity of each test, forgoing the need for a reference test.
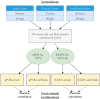
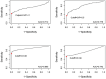
Methods: Here, we estimated and compared the diagnostic sensitivity and specificity of a novel indirect ELISA (iELISA) for ASF virus p30 antibody (Innoceleris LLC.) and the VetAlert™ ASF virus DNA Test Kit (qPCR, Tetracore Inc.) in field samples from Vietnam by assuming that disease status 1) is known and 2) is unknown using a BLCA model. In this cross-sectional study, 398 paired, individual swine serum/oral fluid (OF) samples were collected from 30 acutely ASF-affected farms, 37 chronically ASF-affected farms, and 20 ASF-unaffected farms in Vietnam. Samples were tested using both diagnostic assays. Diagnostic sensitivity was calculated assuming samples from ASF-affected farms were true positives and diagnostic sensitivity by assuming samples from unaffected farms were true negatives. ROC curves were plotted and AUC calculated for each test/sample combination. For comparison, a conditionally dependent, four test/sample combination, three population BLCA model was fit.
Results: When considering all assumed ASF-affected samples, qPCR sensitivity was higher for serum (65.2%, 95% Confidence Interval [CI] 58.1-71.8) and OF (52%, 95%CI 44.8-59.2) compared to the iELISA (serum: 42.9%, 95%CI 35.9-50.1; OF: 33.3%, 95%CI 26.8-40.4). qPCR-serum had the highest AUC (0.895, 95%CI 0.863-0.928). BLCA estimates were nearly identical to those obtained when assuming disease status and were robust to changes in priors. qPCR sensitivity was considerably higher than ELISA in the acutely-affected population, while ELISA sensitivity was higher in the chronically-affected population. Specificity was nearly perfect for all test/sample types.
Discussion: The effect of disease chronicity on sensitivity and specificity could not be well characterized here due to limited data, but future studies should aim to elucidate these trends to understand the best use of virus and antibody detection methods for ASF. Results presented here will help the design of surveillance and control strategies in Vietnam and other countries affected by ASF.
Keywords: African swine fever; Bayesian latent class analysis; ELISA; PCR; Vietnam; diagnostic test; evaluation.
Copyright © 2023 Schambow, Giménez-Lirola, Hanh, Huong, Lan, Trang, Luc, Bo, Chuong, Rauh, Nelson, Mora-Díaz, Rovira, Culhane and Perez.
Conflict of interest statement
RR and WN work for the company Tetracore Inc. LG-L is Associate Professor in the Department of Veterinary Diagnostic and Production Animal Medicine at Iowa State University and founder of Innoceleris LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Montgomery, Eustace R. On a form of swine fever occurring in British East Africa (Kenya Colony). J Comp Pathol Ther. (1921) 34:159–91. 10.1016/S0368-1742(21)80031-4 - DOI
-
- World Organization for Animal Health. African Swine Fever (ASF) – Situation Report 12. OIE - World Organ Anim Health (2022). Available online at: https://www.oie.int/en/document/african-swine-fever-asf-situation-report... (accessed May 18, 2022).
-
- USDA APHIS,. USDA Statement on Confirmation of African Swine Fever in the Dominican Republic. (2021). Available online at: https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa-2021/asf-co... (accessed February 28, 2022).
LinkOut - more resources
Full Text Sources
Research Materials

