Inflammatory Macrophage Interleukin-1β Mediates High-Fat Diet-Induced Heart Failure With Preserved Ejection Fraction
- PMID: 36908663
- PMCID: PMC9998610
- DOI: 10.1016/j.jacbts.2022.08.003
Inflammatory Macrophage Interleukin-1β Mediates High-Fat Diet-Induced Heart Failure With Preserved Ejection Fraction
Abstract
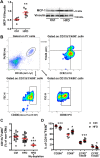
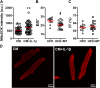
Diabetes mellitus (DM) is a main risk factor for diastolic dysfunction (DD) and heart failure with preserved ejection fraction. High-fat diet (HFD) mice presented with diabetes mellitus, DD, higher cardiac interleukin (IL)-1β levels, and proinflammatory cardiac macrophage accumulation. DD was significantly ameliorated by suppressing IL-1β signaling or depleting macrophages. Mice with macrophages unable to adopt a proinflammatory phenotype were low in cardiac IL-1β levels and were resistant to HFD-induced DD. IL-1β enhanced mitochondrial reactive oxygen species (mitoROS) in cardiomyocytes, and scavenging mitoROS improved HFD-induced DD. In conclusion, macrophage-mediated inflammation contributed to HFD-associated DD through IL-1β and mitoROS production.
Keywords: CCR2, C-C motif chemokine receptor 2; CM, cardiomyocyte; DD, diastolic dysfunction; DM, diabetes mellitus; EF, ejection fraction; FABP4, fatty acid binding protein 4; HF, heart failure; HFD, high-fat diet; HFpEF; HFpEF, heart failure with preserved ejection fraction; IL, interleukin; IL-1β; IL1RA, interleukin 1 receptor antagonist; KO, knockout; MCP, monocyte chemoattractant protein; MyBP-C, myosin binding protein C; TGF, transforming growth factor; TNF, tumor necrosis factor; Timd4, T cell immunoglobulin and mucin domain containing 4; WT, wild-type; diabetes; diastolic dysfunction; inflammation; macrophage; mitoROS, mitochondrial reactive oxygen species; mitochondria.
© 2023 The Authors.
Conflict of interest statement
This project was supported by National Institutes of Health grants R01 HL104025 (Dr Dudley) and R01 HL106592 (Dr Dudley). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






References
-
- Hunt S.A., Abraham W.T., Chin M.H., et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–82. doi: 10.1016/j.jacc.2005.08.022. - DOI - PubMed
-
- Carbone S., Canada J.M., Buckley L.F., et al. Dietary fat, sugar consumption, and cardiorespiratory fitness in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol Basic Trans Science. 2017;2:513–525. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

