Protein-protein interfaces in molecular glue-induced ternary complexes: classification, characterization, and prediction
- PMID: 36908699
- PMCID: PMC9994104
- DOI: 10.1039/d2cb00207h
Protein-protein interfaces in molecular glue-induced ternary complexes: classification, characterization, and prediction
Abstract
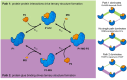
Molecular glues are a class of small molecules that stabilize the interactions between proteins. Naturally occurring molecular glues are present in many areas of biology where they serve as central regulators of signaling pathways. Importantly, several clinical compounds act as molecular glue degraders that stabilize interactions between E3 ubiquitin ligases and target proteins, leading to their degradation. Molecular glues hold promise as a new generation of therapeutic agents, including those molecular glue degraders that can redirect the protein degradation machinery in a precise way. However, rational discovery of molecular glues is difficult in part due to the lack of understanding of the protein-protein interactions they stabilize. In this review, we summarize the structures of known molecular glue-induced ternary complexes and the interface properties. Detailed analysis shows different mechanisms of ternary structure formation. Additionally, we also review computational approaches for predicting protein-protein interfaces and highlight the promises and challenges. This information will ultimately help inform future approaches for rational molecular glue discovery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All authors are employees and stockholders for Amgen, Inc.
Figures










References
-
- Matyskiela M. E. Couto S. Zheng X. Lu G. Hui J. Stamp K. Drew C. Ren Y. Wang M. Carpenter A. Lee C.-W. Clayton T. Fang W. Lu C.-C. Riley M. Abdubek P. Blease K. Hartke J. Kumar G. Vessey R. Rolfe M. Hamann L. G. Chamberlain P. P. SALL4 mediates teratogenicity as a thalidomide-dependent cereblon substrate. Nat. Chem. Biol. 2018;14(10):981–987. doi: 10.1038/s41589-018-0129-x. - DOI - PubMed
-
- Chamberlain P. P. Lopez-Girona A. Miller K. Carmel G. Pagarigan B. Chie-Leon B. Rychak E. Corral L. G. Ren Y. J. Wang M. Riley M. Delker S. L. Ito T. Ando H. Mori T. Hirano Y. Handa H. Hakoshima T. Daniel T. O. Cathers B. E. Structure of the human Cereblon–DDB1–lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 2014;21(9):803–809. doi: 10.1038/nsmb.2874. - DOI - PubMed
-
- Krönke J. Udeshi N. D. Narla A. Grauman P. Hurst S. N. McConkey M. Svinkina T. Heckl D. Comer E. Li X. Ciarlo C. Hartman E. Munshi N. Schenone M. Schreiber S. L. Carr S. A. Ebert B. L. Lenalidomide Causes Selective Degradation of IKZF1 and IKZF3 in Multiple Myeloma Cells. Science. 2014;343(6168):301–305. doi: 10.1126/science.1244851. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

