Blood-brain barrier endothelial cells in neurodegenerative diseases: Signals from the "barrier"
- PMID: 36908787
- PMCID: PMC9998532
- DOI: 10.3389/fnins.2023.1047778
Blood-brain barrier endothelial cells in neurodegenerative diseases: Signals from the "barrier"
Abstract
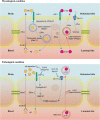
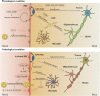
As blood-brain barrier (BBB) disruption emerges as a common problem in the early stages of neurodegenerative diseases, the crucial roles of barrier-type brain endothelial cells (BECs), the primary part of the BBB, have been reported in the pathophysiology of neurodegenerative diseases. The mechanisms of how early vascular dysfunction contributes to the progress of neurodegeneration are still unclear, and understanding BEC functions is a promising start. Our understanding of the BBB has gone through different stages, from a passive diffusion barrier to a mediator of central-peripheral interactions. BECs serve two seemingly paradoxical roles: as a barrier to protect the delicate brain from toxins and as an interface to constantly receive and release signals, thus maintaining and regulating the homeostasis of the brain. Most previous studies about neurodegenerative diseases focus on the loss of barrier functions, and far too little attention has been paid to the active regulations of BECs. In this review, we present the current evidence of BEC dysfunction in neurodegenerative diseases and explore how BEC signals participate in the pathogenesis of neurodegenerative diseases.
Keywords: Alzheimer’s disease; Parkinson’s disease; blood–brain barrier; endothelial cells; neurodegenerative disease; neurovascular unit.
Copyright © 2023 Yuan, Sun, Dong and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

