Altered glutamate-glutamine and amide proton transfer-weighted values in the hippocampus of patients with amnestic mild cognitive impairment: A novel combined imaging diagnostic marker
- PMID: 36908797
- PMCID: PMC9995585
- DOI: 10.3389/fnins.2023.1089300
Altered glutamate-glutamine and amide proton transfer-weighted values in the hippocampus of patients with amnestic mild cognitive impairment: A novel combined imaging diagnostic marker
Abstract
Background and purpose: Early diagnosis of amnestic mild cognitive impairment (aMCI) and timely management to delay the onset of Alzheimer's disease (AD) would benefit patients. Pathological metabolic changes of excitatory/inhibitory neurotransmitters and abnormal protein deposition in the hippocampus of aMCI may provide a new clue to imaging diagnosis. However, the diagnostic performance using these hippocampal metabolite measurements is still unclear. We aimed to quantify right hippocampal glutamate-glutamine (Glx) and gamma-aminobutyric acid (GABA) levels as well as protein-based amide proton transfer-weighted (APTw) signals of patients with aMCI and investigate the diagnostic performance of these metabolites.
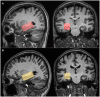
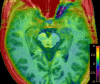
Methods: In this cross-sectional study, 20 patients with aMCI and 20 age- and gender-matched healthy controls (HCs) underwent MEGA Point Resolved Spectroscopy (MEGA-PRESS) and APTw MR imaging at 3 T. GABA+, Glx, and APTw signals were measured in the right hippocampus. The GABA+ levels, Glx levels, Glx/GABA+ ratios, and APTw values were compared between the HCs and aMCI groups using the Mann-Whitney U test. Binary logistic regression and receiver operating characteristic (ROC) curve analyses were used to evaluate MEGA-PRESS and APTw parameters' diagnostic performance.
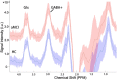
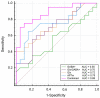
Results: Compared with HCs, patients with aMCI had significantly lower Glx levels in the right hippocampus (7.02 ± 1.41 i.u. vs. 5.81 ± 1.33 i.u., P = 0.018). No significant changes in the GABA+ levels were observed in patients with aMCI (HCs vs. aMCI: 2.54 ± 0.28 i.u. vs. 2.47 ± 0.36 i.u., P = 0.620). In addition, Glx/GABA+ ratios between the two groups (HCs vs. aMCI: 2.79 ± 0.60 vs. 2.37 ± 0.55, P = 0.035) were significantly different. Compared with HCs, patients with aMCI showed higher APTw values in the right hippocampus (0.99 ± 0.26% vs. 1.26% ± 0.28, P = 0.006). The ROC curve analysis showed that Glx, GABA+, Glx/GABA+, and APTw values had an area under the curve (AUC) of 0.72, 0.55, 0.70, and 0.75, respectively, for diagnosing aMCI. In the ROC curve analysis, the AUC of the combination of the parameters increased to 0.88, which is much higher than that observed in the univariate analysis (P < 0.05).
Conclusion: The combination of right hippocampal Glx levels and APTw values improved the diagnostic performance for aMCI, indicating it as a promising combined imaging diagnostic marker. Our study provided a potential imaging diagnostic strategy of aMCI, which may promote early detection of aMCI and facilitate timely intervention to delay the pathological progress toward AD.
Keywords: MEGA-PRESS; amide proton transfer-weighted imaging; amnestic mild cognitive impairment; hippocampus; imaging diagnostic marker.
Copyright © 2023 Chen, Gong, Chen, Xu, Li, Song, Lin, Oeltzschner, Edden, Xia and Wang.
Conflict of interest statement
LL was employed by Philips Healthcare. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

