Strategies for participant retention in long term clinical trials: A participant -centric approaches
- PMID: 36909219
- PMCID: PMC10003583
- DOI: 10.4103/picr.picr_161_21
Strategies for participant retention in long term clinical trials: A participant -centric approaches
Abstract
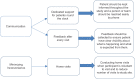
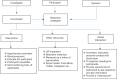
A clinical trial is the most foolproof method to evaluate the efficacy of a new intervention. Successful completion of clinical trials depends on the retention of the participants enrolled. Poor participant retention can lead to significant time and cost burden and have potentially adverse biases on the results. A high retention rate of participants is an important criterion for the validity and credibility of randomized controlled clinical trials. Many long-term trials fail due to low retention of study participants. Efforts at participant retention should start even before the first participant is recruited into the study. Retention is not only the responsibility of the investigators but also all other stakeholders in a clinical trial. In recent years, retention materials, participant camps, and introduction of national study coordinators have helped in improving retention. Quality of the relationship developed between the research staff and the study participant is a key factor for success of any trial. In our experience, in the context of resource-challenged low- and middle-income countries, we have found that it is possible to achieve high retention rates, 95%-100%. The rapport built between the investigating team and the participant plays a vital role in retention. In addition, personalized care, including listening to the participant's problems and enabling to contact investigators or study team at any time of the day, has shown benefits in retention.
Keywords: Clinical trials; retention; stakeholders.
Copyright: © 2022 Perspectives in Clinical Research.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources