Metabolic crosstalk between stromal and malignant cells in the bone marrow niche
- PMID: 36909665
- PMCID: PMC9996235
- DOI: 10.1016/j.bonr.2023.101669
Metabolic crosstalk between stromal and malignant cells in the bone marrow niche
Abstract
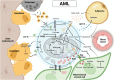
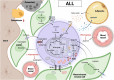
Bone marrow is the primary site of blood cell production in adults and serves as the source of osteoblasts and osteoclasts that maintain bone homeostasis. The medullary microenvironment is also involved in malignancy, providing a fertile soil for the growth of blood cancers or solid tumors metastasizing to bone. The cellular composition of the bone marrow is highly complex, consisting of hematopoietic stem and progenitor cells, maturing blood cells, skeletal stem cells, osteoblasts, mesenchymal stromal cells, adipocytes, endothelial cells, lymphatic endothelial cells, perivascular cells, and nerve cells. Intercellular communication at different levels is essential to ensure proper skeletal and hematopoietic tissue function, but it is altered when malignant cells colonize the bone marrow niche. While communication often involves soluble factors such as cytokines, chemokines, and growth factors, as well as their respective cell-surface receptors, cells can also communicate by exchanging metabolic information. In this review, we discuss the importance of metabolic crosstalk between different cells in the bone marrow microenvironment, particularly concerning the malignant setting.
Keywords: Bone marrow; Bone metastasis; Cell metabolism; Cellular communication; Leukemia; Stromal cells.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Allaman I., et al. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34(2):76–87. - PubMed
LinkOut - more resources
Full Text Sources

