Efficacies of Potential Probiotic Candidates Isolated from Traditional Fermented Korean Foods in Stimulating Immunoglobulin A Secretion
- PMID: 36909859
- PMCID: PMC9998188
- DOI: 10.5851/kosfa.2023.e2
Efficacies of Potential Probiotic Candidates Isolated from Traditional Fermented Korean Foods in Stimulating Immunoglobulin A Secretion
Abstract
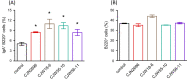
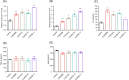
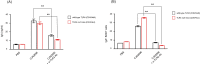
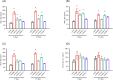
The aim of this study was to evaluate efficacies of selected lactic acid bacteria (LAB) in inducing immunoglobulin A (IgA) secretion. Twenty-five different LAB isolated from traditional fermented Korean foods were characterized for their probiotic properties and screened to identify those that could stimulate lamina propria cells (LPCs) from Peyer's patch to secret IgA in vitro. Among them, four strains (Lactiplantibacillus plantarum CJW55-10, Lactiplantibacillus pentosus CJW18-6, L. pentosus CJW56-11, and Pediococcus acidilactici CJN2696) were found to be strong IgA inducers. The number of IgA positive B cells and soluble IgA level were increased when LPCs were co-cultured with these LAB. Expression levels of toll-like receptor (TLR) such as TLR2 and TLR4 and secretion of interleuckin-6 were augmented in LPCs treated with these LAB. Further, we determined whether oral intake of these LAB enhanced IgA production in vivo. After one-week of daily oral administration, these LAB feed mice increased mucosal IgA and serum IgA. In conclusion, selected strains of LAB could induce systemic IgA secretion by activating lamina propria B cells in Peyer's patch and oral intake of selected strains of LAB can enhance systemic immunity by inducing mucosal IgA secretion.
Keywords: Peyer’s patch; immunoglobulin A; interleukin-6; lactic acid bacteria; toll-like receptor.
© Korean Society for Food Science of Animal Resources.
Conflict of interest statement
Hyun Sun Yun is currently employed by the CJ CheilJedang Corporation (Korea).
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

