Crystal structures of di-μ-chlorido-bis-({(E)-5-(ethyl-amino)-4-methyl-2-[(pyridin-2-yl)diazen-yl]phen-o-lato}copper(II)) and chlorido-bis-(1,10-phen-anthroline)copper(II) chloride tetra-hydrate
- PMID: 36909989
- PMCID: PMC9993926
- DOI: 10.1107/S205698902300138X
Crystal structures of di-μ-chlorido-bis-({(E)-5-(ethyl-amino)-4-methyl-2-[(pyridin-2-yl)diazen-yl]phen-o-lato}copper(II)) and chlorido-bis-(1,10-phen-anthroline)copper(II) chloride tetra-hydrate
Abstract
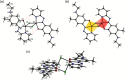
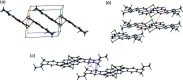
The dark-red title complex crystallized from an equimolar methanol solution of (E)-5-(ethyl-amino)-4-methyl-2-[(pyridin-2-yl)diazen-yl]phenol and CuCl2(phen) (phen = 1,10-phenanthroline) as a centrosymmetric dimer, [CuCl(C14H15N4O)]2. The Cu atoms are bridged by two Cl ligands and have a slightly distorted square-pyramidal coordination, where two N atoms from the azo and the pyridine moieties, a phenolic O and a Cl atom comprise the base and the other Cl occupies the apex position. The apical Cu-Cl bond, 2.6192 (4) Å, is longer than the basal one, 2.2985 (3) Å, due to Jahn-Teller distortion. The dimers are associated via weak inter-molecular hydrogen bonds and π-π stacking inter-actions between phenyl and pyridine rings. A monomeric by-product of the same reaction, [CuCl(phen)2]Cl·4H2O, has a trigonal-bipyramidal coordination of Cu with equatorial Cl ligand, and extensive outer-sphere disorder. In the structure of 4, the packing of cations leaves continuous channels containing disordered Cl- anions and solvent mol-ecules. The identity of the solvent (water or a water/methanol mixture) was not certain. The disordered anion/solvent regions comprise 28% of the unit-cell volume. The disorder was approximated by five partly occupied positions of the Cl- anion and ten positions of O atoms with a total occupancy of 3, giving a total of 48 electrons per asymmetric unit, in agreement with the integral electron density of 47.8 electrons in the disordered region, as was estimated using the BYPASS-type solvent-masking program [van der Sluis & Spek (1990). Acta Cryst. A46, 194-201].
Keywords: X-ray crystallography; chlorido bridge; copper(II) complex; supramolecular features.
© Meier et al. 2023.
Figures


References
-
- Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J. & Verschoor, G. C. (1984). J. Chem. Soc. Dalton Trans. pp. 1349–1356.
-
- Anderson, O. P. (1975). Inorg. Chem. 14, 730–734.
-
- Bruker (2016). SAINT. Bruker Nano Inc., Madison, Wisconsin, USA.
-
- Bruker (2021). APEX4. Bruker Nano Inc., Madison, Wisconsin, USA.
-
- Bunton, C. A., Minch, M. J. & Wolfe, B. B. (1974). J. Am. Chem. Soc. 96, 3267–3275.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
