Crystal structures of the sulfones of 2,3-diphenyl-2,3-di-hydro-4 H-1,3-benzo-thia-zin-4-one and 2,3-diphenyl-2,3-di-hydro-4 H-pyrido[3,2- e][1,3]thia-zin-4-one
- PMID: 36909997
- PMCID: PMC9993925
- DOI: 10.1107/S2056989023001524
Crystal structures of the sulfones of 2,3-diphenyl-2,3-di-hydro-4 H-1,3-benzo-thia-zin-4-one and 2,3-diphenyl-2,3-di-hydro-4 H-pyrido[3,2- e][1,3]thia-zin-4-one
Abstract
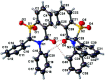
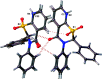
The title sulfones, 2,3-diphenyl-2,3-di-hydro-4H-1,3-benzo-thia-zine-1,1,4-trione, C20H15NO3S, and 2,3-diphenyl-2,3-di-hydro-4H-pyrido[3,2-e][1,3]thia-zine-1,1,4-trione, C19H14N2O3S, crystallize in space group P21/n with two mol-ecules in each of the asymmetric units and have almost identical unit cells and extended structures. In both structures, the thia-zine rings exhibit a screw-boat pucker. The inter-molecular inter-actions observed are C-H⋯O-type hydrogen bonds and parallel partial π-π stacking between the fused aromatic rings (benzo- or pyrido-) of the core of the mol-ecules within each asymmetric unit, and also connecting to mol-ecules with translational periodicity in the a-axis direction in what can be described as columns (two per asymmetric unit) of stacked mol-ecules with alternating chirality. The pendant phenyl groups of both mol-ecules do not participate in aromatic ring inter-actions.
Keywords: 1,3-thiazin-4-one; S-oxidation; crystal structure; screw-boat pucker; sulfone.
© Yennawar et al. 2023.
Figures



References
-
- Bruker (2001). SMART. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2016). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cannon, K., Gandla, D., Lauro, S., Silverberg, L., Tierney, J. & Lagalante, A. (2015). Int. J. Chem. 7, 73–84.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
