Anatomic lung resection after immune checkpoint inhibitors for initially unresectable advanced-staged non-small cell lung cancer: a retrospective cohort analysis
- PMID: 36910122
- PMCID: PMC9992580
- DOI: 10.21037/jtd-22-704
Anatomic lung resection after immune checkpoint inhibitors for initially unresectable advanced-staged non-small cell lung cancer: a retrospective cohort analysis
Abstract
Background: Patients with initially unresectable advanced non-small cell lung cancer (NSCLC) might experience prolonged responses under immune checkpoint inhibitors (ICIs). In this setting, Multidisciplinary Tumor Board (MTB) seldomly suggest surgical resection of the primary tumor with the ultimate goal to eradicate macroscopic residual disease. Our objective was to report the perioperative outcomes of patients who underwent anatomic lung resection in these infrequent circumstances.
Methods: We set a retrospective multicentric single arm study, including all patients with advanced-staged initially unresectable NSCLC (stage IIIB to IVB) who received systemic therapy including ICIs and eventually anatomical resection of the primary tumor in 10 French thoracic surgery units from January 2016 to December 2020. Coprimary endpoints were in-hospital mortality and morbidity. Secondary endpoints were the rate of complete resection of the pulmonary disease, major pathologic response, risk factors associated with post-operative complications, and overall survival.
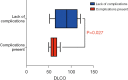
Results: Twenty-one patients (median age 64, female 62%) were included. Eighteen patients (86%) progressed after first line chemotherapy and received second line ICI. The median time between diagnosis and surgery was 22 months [interquartile range (IQR) 18-35 months]. Minimally-invasive approach was used in 10 cases (48%), with half of these requiring conversion to open thoracotomy. Nine patients (43%) presented early post-operative complications, and one patient died from broncho-pleural fistula one month after surgery. Rates of complete resection of the pulmonary disease and major pathologic response were 100% and 43%, respectively. In univariable analysis, diffusing capacity for carbon monoxide (DLCO) was the only factor associated with the occurrence of postoperative complications (P=0.027). After a median follow-up of 16.0 months after surgery (IQR, 12.0-30.0 months), 19 patients (90%) were still alive.
Conclusions: Anatomic lung resections appear to be a reasonable option for initially unresectable advanced NSCLC experiencing prolonged response under ICIs. Nonetheless, minimally invasive techniques have a low applicability and post-operative complications remains higher in patients who had lower DLCO values. The late timing of surgery may also contribute to complications.
Keywords: Non-small cell lung cancers (NSCLCs); anatomic lung resection; immune checkpoint inhibitors (ICIs).
2023 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-704/coif). The authors have no conflicts of interest to declare.
Figures
References
LinkOut - more resources
Full Text Sources