Composition and biodiversity of soil and root-associated microbiome in Vitis vinifera cultivar Lambrusco distinguish the microbial terroir of the Lambrusco DOC protected designation of origin area on a local scale
- PMID: 36910169
- PMCID: PMC9992870
- DOI: 10.3389/fmicb.2023.1108036
Composition and biodiversity of soil and root-associated microbiome in Vitis vinifera cultivar Lambrusco distinguish the microbial terroir of the Lambrusco DOC protected designation of origin area on a local scale
Abstract
Introduction: Wines produced from the same grape cultivars but in different locations possess distinctive qualities leading to different consumer's appreciation, preferences, and thus purchase choices. Here, we explore the possible importance of microbiomes at the soil-plant interface as a determinant of the terroir properties in grapevine production, which confer specific growth performances and wine chemo-sensory properties at the local scale.
Methods: In particular, we investigated the variation in microbial communities associated with the roots of Vitis vinifera cultivar Lambrusco, as well as with surrounding bulk soils, in different vineyards across the "Consorzio Tutela Lambrusco DOC" protected designation of origin area (PDO, Emilia Romagna, Italy), considering viticultural sites located both inside and outside the consortium in two different seasons (June and November 2021).
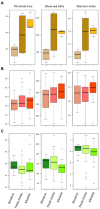
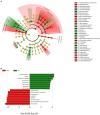
Results: According to our findings, rhizospheric and soil microbiomes show significant structural differences in relation to the sampling site, regardless of seasonality, while endophytic microbiomes seem to be completely unaffected by such variables. Furthermore, a deeper insight into the microbial terroir of PDO areas highlighted the presence of some rhizospheric microorganisms enriched inside the consortium and characterizing the PDO regardless of both sampling season and farming strategy. These include Bacillus, Paenibacillus, and Azospirillum, which are all well-known plant growth-promoting bacteria.
Discussion: Taken together, our results suggest a connection between soil and root microbiomes of V. vinifera cultivar Lambrusco and the local designation of origin, emphasizing the potential role of PDO-enriched plant growth-promoting bacteria in vine growing and final quality of the Lambrusco DOC wine.
Keywords: Vitis vinifera; microbial terroir; microbiomes; plant growth-promoting bacteria; rhizosphere.
Copyright © 2023 Nanetti, Palladino, Scicchitano, Trapella, Cinti, Fabbrini, Cozzi, Accetta, Tassini, Iannaccone, Candela and Rampelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Aguilar O. M., Gobbi A., Browne P. D., Ellegaard-Jensen L., Hansen L. H., Semorile L., et al. . (2020). Influence of vintage, geographic location and cultivar on the structure of microbial communities associated with the grapevine rhizosphere in vineyards of San Juan Province, Argentina. PLoS One 15:e0243848. doi: 10.1371/journal.pone.0243848, PMID: - DOI - PMC - PubMed
-
- Anderson M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x - DOI
-
- Ansari F. A., Ahmad I., Pichtel J. (2019). Growth stimulation and alleviation of salinity stress to wheat by the biofilm forming Bacillus pumilus strain FAB10. Appl. Soil Ecol. 143, 45–54. doi: 10.1016/j.apsoil.2019.05.023 - DOI
-
- Arzanesh M. H., Alikhani H. A., Khavazi K., Rahimian H. A., Miransari M. (2011). Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 27, 197–205. doi: 10.1007/s11274-010-0444-1 - DOI
LinkOut - more resources
Full Text Sources

