Efficacy of first dose of covid-19 vaccine versus no vaccination on symptoms of patients with long covid: target trial emulation based on ComPaRe e-cohort
- PMID: 36910458
- PMCID: PMC9978748
- DOI: 10.1136/bmjmed-2022-000229
Efficacy of first dose of covid-19 vaccine versus no vaccination on symptoms of patients with long covid: target trial emulation based on ComPaRe e-cohort
Abstract
Objective: To evaluate the effect of covid-19 vaccination on the severity of symptoms in patients with long covid.
Design: Target trial emulation based on ComPaRe e-cohort.
Data source: ComPaRe long covid cohort, a nationwide e-cohort (ie, a cohort where recruitment and follow-up are performed online) of patients with long covid, in France.
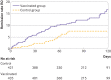
Methods: Adult patients (aged ≥18 years) enrolled in the ComPaRe cohort before 1 May 2021 were included in the study if they reported a confirmed or suspected SARS-CoV-2 infection, symptoms persistent for >3 weeks after onset, and at least one symptom attributable to long covid at baseline. Patients who received a first covid-19 vaccine injection were matched with an unvaccinated control group in a 1:1 ratio according to their propensity scores. Number of long covid symptoms, rate of complete remission of long covid, and proportion of patients reporting an unacceptable symptom state at 120 days were recorded.
Results: 910 patients were included in the analyses (455 in the vaccinated group and 455 in the control group). By 120 days, vaccination had reduced the number of long covid symptoms (mean 13.0 (standard deviation 9.4) in the vaccinated group v 14.8 (9.8) in the control group; mean difference -1.8, 95% confidence interval -3.0 to -0.5) and doubled the rate of patients in remission (16.6% v 7.5%, hazard ratio 1.93, 95% confidence interval 1.18 to 3.14). Vaccination reduced the effect of long covid on patients' lives (mean score on the impact tool 24.3 (standard deviation 16.7) v 27.6 (16.7); mean difference -3.3, 95% confidence interval -5.7 to -1.0) and the proportion of patients with an unacceptable symptom state (38.9% v 46.4%, risk difference -7.4%, 95% confidence interval -14.5% to -0.3%). In the vaccinated group, two (0.4%) patients reported serious adverse events requiring admission to hospital.
Conclusion: In this study, covid-19 vaccination reduced the severity of symptoms and the effect of long covid on patients' social, professional, and family lives at 120 days in those with persistent symptoms of infection.
Keywords: COVID-19.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Impact of covid-19 vaccination on long covid.BMJ Med. 2023 Feb 1;2(1):e000470. doi: 10.1136/bmjmed-2022-000470. eCollection 2023. BMJ Med. 2023. PMID: 36936263 Free PMC article. No abstract available.
-
Nachträgliche Impfung bei Long-COVID lindert offenbar Symptome.MMW Fortschr Med. 2023 Apr;165(7):19. doi: 10.1007/s15006-023-2552-5. MMW Fortschr Med. 2023. PMID: 37016218 Free PMC article. German. No abstract available.
References
-
- Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. London, UK; 2021. [Accessed 02 Sep 2021].
LinkOut - more resources
Full Text Sources
Miscellaneous
